All Photos(1)
About This Item
Empirical Formula (Hill Notation):
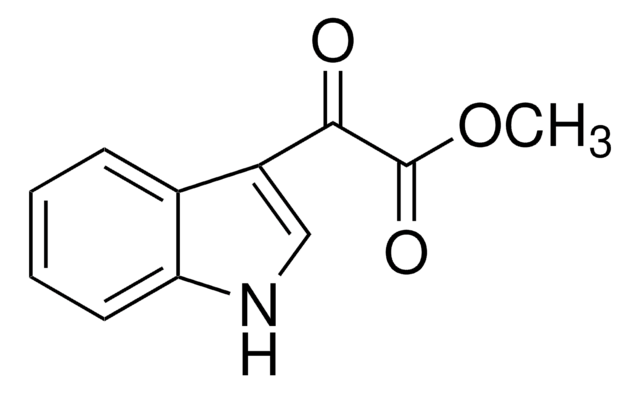
C12H11NO3
CAS Number:
Molecular Weight:
217.22
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
96-100 °C (lit.)
SMILES string
COC(=O)C(=O)c1cn(C)c2ccccc12
InChI
1S/C12H11NO3/c1-13-7-9(11(14)12(15)16-2)8-5-3-4-6-10(8)13/h3-7H,1-2H3
InChI key
SBHIWUQNUXJUMN-UHFFFAOYSA-N
General description
Methyl (1-methylindolyl)-3-glyoxylate can be prepared from 1-methylindole.
Application
- Reactant for synthesis of sotrastaurin analogs
- Reactant for preparation of protein kinase C (PKC) inhibitors
- Reactant for preparation of indolyl diols
Methyl (1-methylindolyl)-3-glyoxylate may be used to synthesize 3-[(1-methyl)-3-indolyl]-4-(3-indolyl)-1H-pyrrole-2,5-dione and 3,4-[(1-methyl)-3-indolyl]-1H-pyrrole-2,5-dione.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A New, Efficient Method for the Synthesis of Bisindolylmaleimides.
Margaret M. Faul et al.
The Journal of organic chemistry, 63(17), 6053-6058 (2001-10-24)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service