510300
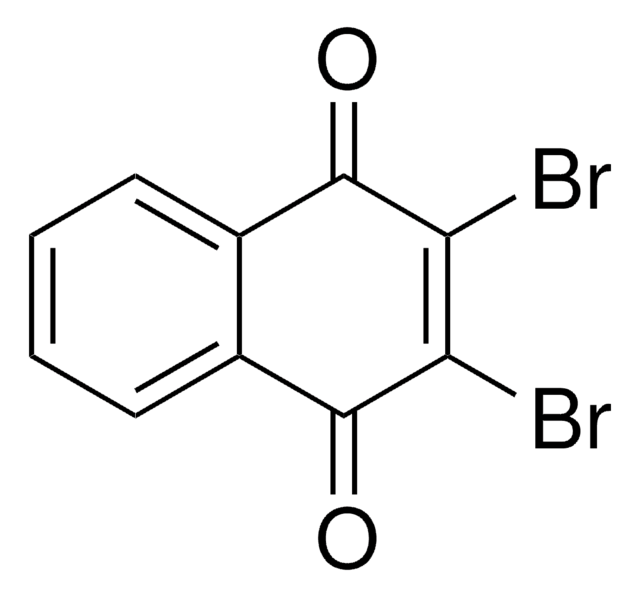
2-Bromo-1,4-naphthoquinone
98%
Synonym(s):
2-Bromo-1,4-dihydronaphthalene-1,4-dione, 2-Bromo-1,4-naphthalenedione, 2-Bromo-p-naphthoquinone, 3-Bromo-1,4-naphthoquinone, 3-Bromonaphthoquinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H5BrO2
CAS Number:
Molecular Weight:
237.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
131-133 °C (lit.)
SMILES string
BrC1=CC(=O)c2ccccc2C1=O
InChI
1S/C10H5BrO2/c11-8-5-9(12)6-3-1-2-4-7(6)10(8)13/h1-5H
InChI key
KJOHPBJYGGFYBJ-UHFFFAOYSA-N
General description
2-Bromo-1,4-naphthoquinone (BrQ) is a 1,4-naphthoquinone derivative that can be prepared by the bromination of 1-naphthol using N-bromosuccinimide. Its efficacy as an antifeedant against the cabbage looper, Trichoplusia ni has been evaluated.2 The reaction of BrQ with xanthene under photochemical conditions has been investigated. A report suggests that the replacement of 2-methyl-1,4-naphthoquinone with BrQ enhances its ability to produce hydrogen peroxide which can be employed for cancer treatment.
Application
2-Bromo-1,4-naphthoquinone may be used in the preparation of:
- benzo[f]indolequinones
- 2-azido-1,4-naphthoquinone
- 2-allyl-3-bromo-1,4-naphthoquinone
- 2-(3-indolyl)-1,4-naphthoquinones
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The Photochemical Reaction of 1,4-Naphthoquinone Derivatives with Hydrogen Donors.
Maruyama K and Arakawa S.
Bulletin of the Chemical Society of Japan, 47(8), 1960-1966 (1974)
Padmakar A Suryavanshi et al.
Organic & biomolecular chemistry, 8(15), 3426-3436 (2010-06-10)
The CAN-catalyzed three-component between reaction between primary amines, beta-dicarbonyl compounds and naphthoquinones or 2-bromonaphthoquinones afforded, respectively, 5-hydroxybenzo[g]indoles and benzo[f]indole-4,9-diones, the former of which were transformed into tetracyclic azepino[1,2-a]benzo[g]indole systems through a gamma-alkylation/ring-closing metathesis sequence.
Tiago T Guimarães et al.
European journal of medicinal chemistry, 63, 523-530 (2013-03-29)
Continuing our screening program for novel anti-parasite compounds, we synthesized seven 1,4-naphthoquinones coupled to 1,2,3-triazoles, five nor-β-lapachone-based 1,2,3-triazoles and ten α-lapachone-based 1,2,3-triazoles. These and other naphthoquinonoid compounds were evaluated for their activity against promastigote forms of antimony-sensitive and -resistant strains
Stereoselective synthesis of deoxy analogues of the 3C-protease inhibitor thysanone.
Brimble MA and Elliott RJR
Tetrahedron, 58(1), 183-189 (2002)
F S Graciani et al.
Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas, 45(8), 701-710 (2012-05-16)
Apatone™, a combination of menadione (2-methyl-1,4-naphthoquinone, VK3) and ascorbic acid (vitamin C, VC) is a new strategy for cancer treatment. Part of its effect on tumor cells is related to the cellular pro-oxidative imbalance provoked by the generation of hydrogen
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service