394815
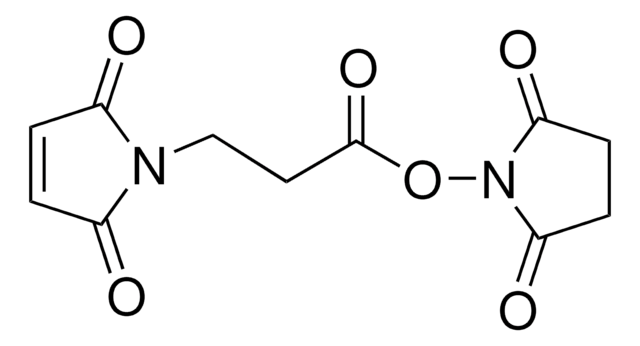
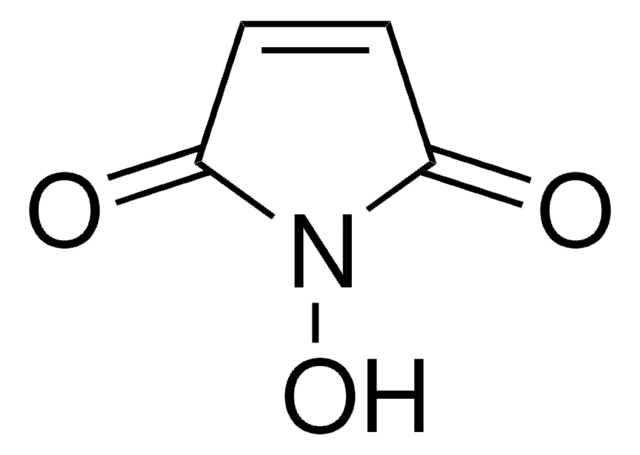
N-Maleoyl-β-alanine
97%
Synonym(s):
3-Maleimidopropionic acid, N-(2-Carboxyethyl)maleimide
About This Item
Recommended Products
Quality Level
Assay
97%
form
powder
greener alternative product score
old score: 18
new score: 7
Find out more about DOZN™ Scoring
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Inherently Safer Chemistry for Accident Prevention
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
103-106 °C (lit.)
greener alternative category
storage temp.
2-8°C
SMILES string
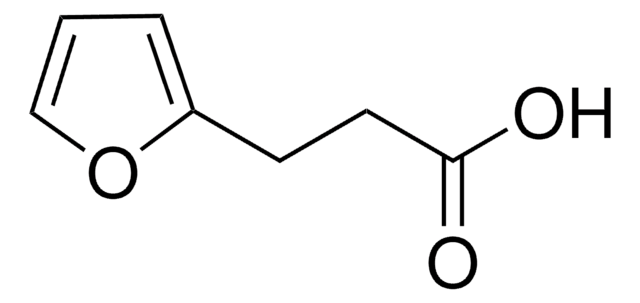
OC(=O)CCN1C(=O)C=CC1=O
InChI
1S/C7H7NO4/c9-5-1-2-6(10)8(5)4-3-7(11)12/h1-2H,3-4H2,(H,11,12)
InChI key
IUTPJBLLJJNPAJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- To decrease the biotin binding affinity of Avd(S16C) (avidin with a single point mutation S16C).
- As a side chain reactive agent to modify tryptic peptides that result in mass shifts indicating the presence of cysteine residues.
- To preblock Xenopus laevis oocytes for exposed cysteines used as an expression system in the study of conformational changes in cASIC1a Receptors.
- As a protective agent for keratin fiber in high temperature process.
- As a non-cleavable maleimido moiety during the synthesis of tetrawalled molecular umbrella-octaarginine conjugates.
- Synthesis of organotin carboxylates of N-Maleoyl-β-alanine.
- To functionalize the gold surfaces to interact with cysteine-modified peptide.
- Preparation of cross-linked dextran–poly(ethylene glycol) hydrogel substrate.
Packaging
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service