476412
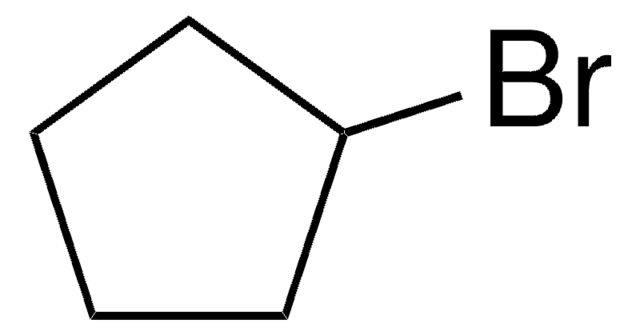
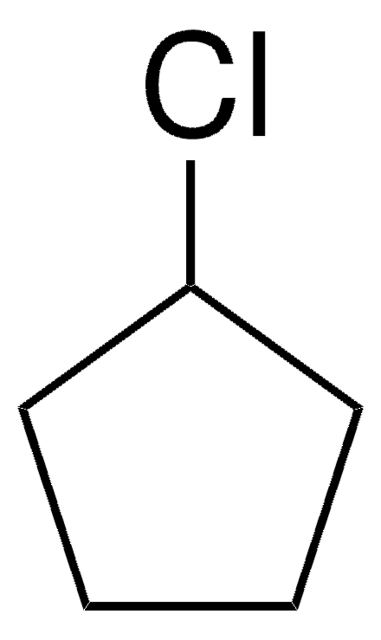
Iodocyclopentane
97%
Synonym(s):
Cyclopentyl iodide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C5H9I
CAS Number:
Molecular Weight:
196.03
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
contains
copper as stabilizer
refractive index
n20/D 1.549 (lit.)
bp
77 °C/45 mmHg (lit.)
density
1.695 g/mL at 25 °C (lit.)
SMILES string
IC1CCCC1
InChI
1S/C5H9I/c6-5-3-1-2-4-5/h5H,1-4H2
InChI key
PCEBAZIVZVIQEO-UHFFFAOYSA-N
Application
Iodocyclopentane may be used in the synthesis of 4-chloro-3′-((2-cyclopentyl-1-oxo-1,2,3,4-tetrahydroisoquinolin-6- yloxy)methyl)biphenyl-3-carboxylic acid and N-(3-methoxyphenethyl)cyclopentanamine.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
125.6 °F - closed cup
Flash Point(C)
52 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Resonance Raman study of the A-band short-time photodissociation dynamics of axial and equatorial conformers of iodocyclopentane.
Zheng X, et al.
J. Chem. Phys., 111(24), 11034-11043 (1999)
Raveendra-Panickar Dhanya et al.
Journal of medicinal chemistry, 54(1), 342-353 (2010-12-16)
The modification of 3'-((2-cyclopentyl-6,7-dimethyl-1-oxo-2,3-dihydro-1H-inden-5-yloxy)methyl)biphenyl-4-carboxylic acid (BINA, 1) by incorporating heteroatoms into the structure and replacing the cyclopentyl moiety led to the development of new mGluR2 positive allosteric modulators (PAMs) with optimized potency and superior druglike properties. These analogues are more
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service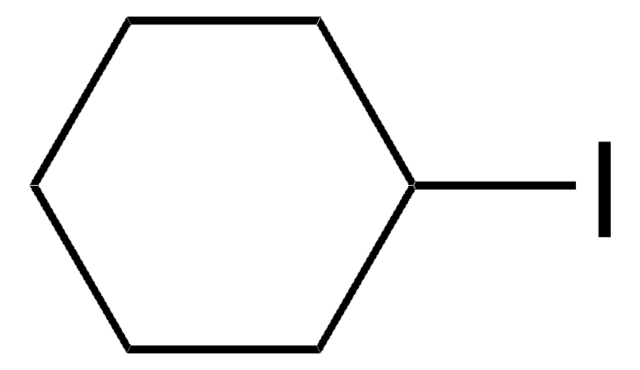

![(R)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether technical grade](/deepweb/assets/sigmaaldrich/product/structures/275/930/055f0504-bc37-431a-b746-95d9fc72ba2d/640/055f0504-bc37-431a-b746-95d9fc72ba2d.png)