238244
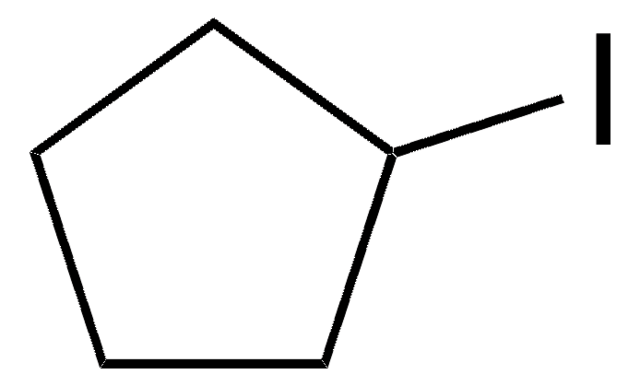
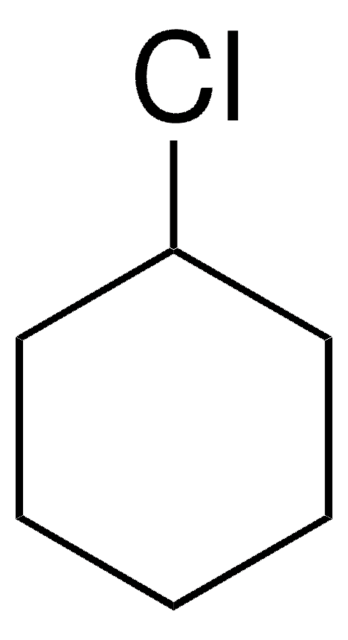
Iodocyclohexane
98%
Synonym(s):
Cyclohexyl iodide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H11I
CAS Number:
Molecular Weight:
210.06
Beilstein:
1900797
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.5441 (lit.)
bp
80-81 °C/20 mmHg (lit.)
density
1.624 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
IC1CCCCC1
InChI
1S/C6H11I/c7-6-4-2-1-3-5-6/h6H,1-5H2
InChI key
FUCOMWZKWIEKRK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The photodissociation dynamics of iodocyclohexane has been studied using velocity map imaging. Nickel catalyzed coupling of iodocyclohexane with 1-octyne has been investigated.
Application
Iodocyclohexane has been employed as reagent in demethylation of aryl methyl ethers in DMF under reflux condition.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
158.0 °F - closed cup
Flash Point(C)
70 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D K Zaouris et al.
The Journal of chemical physics, 135(9), 094312-094312 (2011-09-15)
The photodissociation dynamics of iodocyclohexane has been studied using velocity map imaging following excitation at many wavelengths within its A-band (230 ≤ λ ≤ 305 nm). This molecule exists in two conformations (axial and equatorial), and one aim of the
An efficient method for demethylation of aryl methyl ethers.
Zuo L, et al.
Tetrahedron Letters, 49(25), 4054-4056 (2008)
Jun Yi et al.
Angewandte Chemie (International ed. in English), 52(47), 12409-12413 (2013-10-12)
A nicked reaction: The title reaction of terminal alkynes with non-activated secondary alkyl iodides and bromides was accomplished for the first time. This reaction provides a new and practical approach for the synthesis of substituted alkynes (see scheme; cod=cyclo-1,5-octadiene).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service