471623
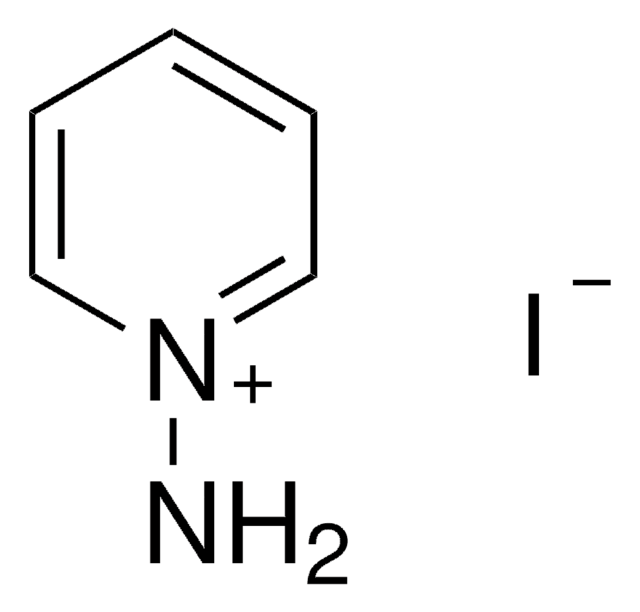
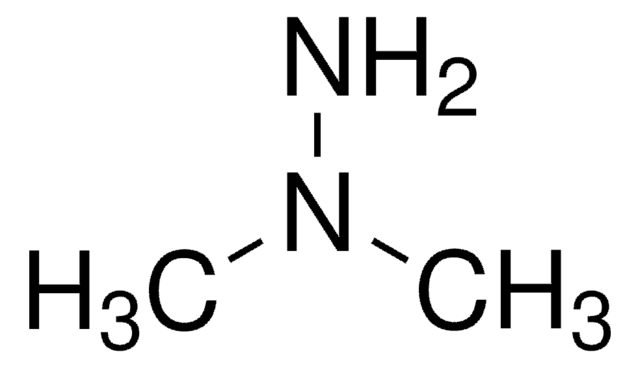
1,1,1-Trimethylhydrazinium iodide
97%
Synonym(s):
1,1,1-Trimethylhydrazine iodide, N,N,N-Trimethylhydrazinium iodide, Trimethylhydrazonium iodide (6CI)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NN(CH3)3I
CAS Number:
Molecular Weight:
202.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
225-230 °C (dec.) (lit.)
SMILES string
[I-].C[N+](C)(C)N
InChI
1S/C3H11N2.HI/c1-5(2,3)4;/h4H2,1-3H3;1H/q+1;/p-1
InChI key
HLNPDVKKRBWQAA-UHFFFAOYSA-M
General description
1,1,1-Trimethylhydrazinium iodide ([(CH3)3N-NH2]I, TMHI) is widely employed as a vicarious nucleophilic substitution (VNS) reagent for various aromatic amination reactions. On reaction with silver salt, it affords the following salts: nitrate (([(CH3)3N-NH2][NO3]), perchlorate ([(CH3)3N-NH2][ClO4]), azide ([(CH3)3N-NH2][N3]), 5-amino-1H-tetrazolate ([(CH3)3N-NH2][H2N-CN4]) and sulfate ([(CH3)3N-NH2]2[SO4].2H2O).
Application
1,1,1-Trimethylhydrazinium iodide (TMHI) may be employed for the amination of 1-X-3,5-dinitrobenzenes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Carles Miró Sabaté et al.
Chemistry, an Asian journal, 7(9), 2080-2089 (2012-06-29)
1,1,1-Trimethylhydrazinium iodide ([(CH(3))(3)N-NH(2)]I, 1) was reacted with a silver salt to form the corresponding nitrate ([(CH(3))(3)N-NH(2)][NO(3)], 2), perchlorate ([(CH(3))(3)N-NH(2)][ClO(4)], 3), azide ([(CH(3))(3)N-NH(2)][N(3)], 4), 5-amino-1H-tetrazolate ([(CH(3))(3)N-NH(2)][H(2)N-CN(4)], 5), and sulfate ([(CH(3))(3)N-NH(2)](2)[SO(4)]·2H(2)O, 6·2H(2)O) salts. The metathesis reaction of compound 6·2H(2)O with barium salts
Vladimir V Rozhkov et al.
The Journal of organic chemistry, 68(6), 2498-2501 (2003-03-15)
The amination of 1-X-3,5-dinitrobenzenes via the vicarious nucleophilic substitution of hydrogen (VNS) with 1,1,1-trimethylhydrazinium iodide (TMHI) in the presence of t-BuOK or NaOMe in DMSO was studied. It was observed (when X = OMe, OCH(2)CF(3), OCH(2)CF(2)CF(2)H, OPh) that the amination
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service