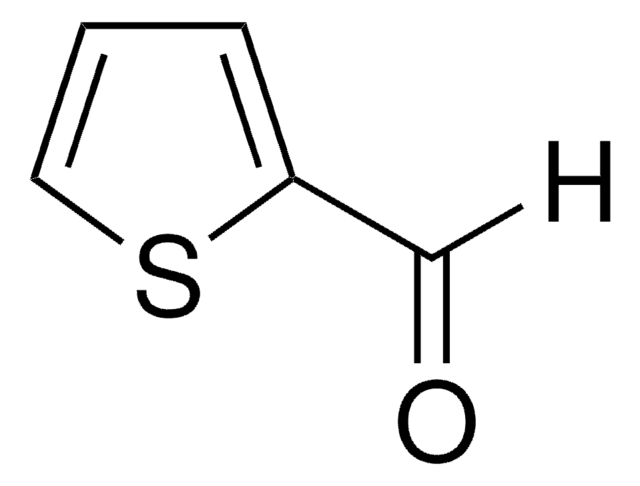
429880
2,5-Thiophenedicarboxaldehyde
99%
Synonym(s):
2,5-Diformylthiophene, 2,5-Thienodicarboxaldehyde, 2,5-Thiophenedial, Thiophene-2,5-dialdehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4O2S
CAS Number:
Molecular Weight:
140.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
115-117 °C (lit.)
SMILES string
[H]C(=O)c1ccc(s1)C([H])=O
InChI
1S/C6H4O2S/c7-3-5-1-2-6(4-8)9-5/h1-4H
InChI key
OTMRXENQDSQACG-UHFFFAOYSA-N
General description
2,5-Thiophenedicarboxaldehyde can be prepared from 2,5-bis(chloromethyl)thiophene by the application of Kröhnke′s method.
Application
2,5-Thiophenedicarboxaldehyde may be employed in the following studies:
- Asymmetric synthesis of bis-homoallylic alcohols.
- Synthesis of new symmetrical arylene bisimide derivatives.
- As dialdehyde monomer in the synthesis of silicon-containing poly(p-phenylenevinylene)-related copolymers having uniform p-conjugated segment regulated by organosilicon units.
- Synthesis of 2,5-bis[2-(5-N-isopropylamidino)benzimidazoyl]thiophene hydrochloride.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xiaoming Li et al.
Frontiers in oncology, 8, 354-354 (2018-10-16)
RNA interference (RNAi) is a biological process through which gene expression can be inhibited by RNA molecules with high selectivity and specificity, providing a promising tool for tumor treatment. Two types of molecules are often applied to inactivate target gene
M Del Poeta et al.
Antimicrobial agents and chemotherapy, 42(10), 2495-2502 (1998-10-03)
Twenty analogues of pentamidine, 7 primary metabolites of pentamidine, and 30 dicationic substituted bis-benzimidazoles were screened for their inhibitory and fungicidal activities against Candida albicans and Cryptococcus neoformans. A majority of the compounds had MICs at which 80% of the
The Preparation of 2, 5-Thiophenedicarboxaldehyde.
Sone T.
Bulletin of the Chemical Society of Japan, 37(8), 1197-1200 (1964)
Marzena Grucela-Zajac et al.
The journal of physical chemistry. C, Nanomaterials and interfaces, 118(24), 13070-13086 (2014-06-27)
New symmetrical arylene bisimide derivatives formed by using electron-donating-electron-accepting systems were synthesized. They consist of a phthalic diimide or naphthalenediimide core and imine linkages and are end-capped with thiophene, bithiophene, and (ethylenedioxy)thiophene units. Moreover, polymers were obtained from a new
Guang-Ming Chen et al.
The Journal of organic chemistry, 64(3), 721-725 (2001-10-25)
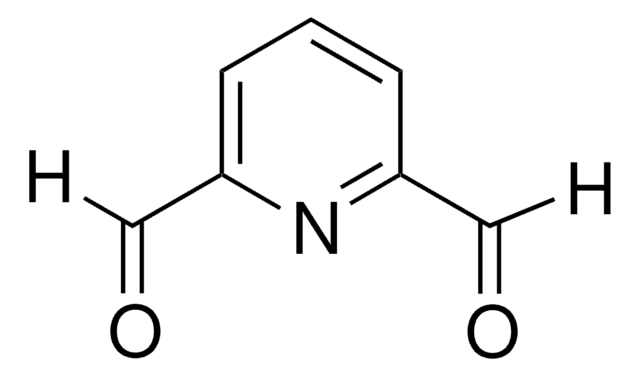
Asymmetric reduction of 2,6-diacylpyridines with B-chlorodiisopinocampheylborane provides the corresponding C(2)-symmetric diols in very high de and ee. Asymmetric allylboration of 2,6-pyridinedicarboxaldehyde and 2,5-thiophenedicarboxaldehyde provides the corresponding bis-homoallylic alcohols in very high de and ee. These optically pure diols were converted
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)