440833
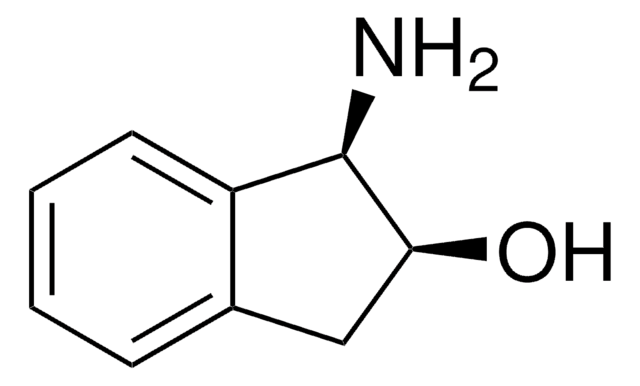
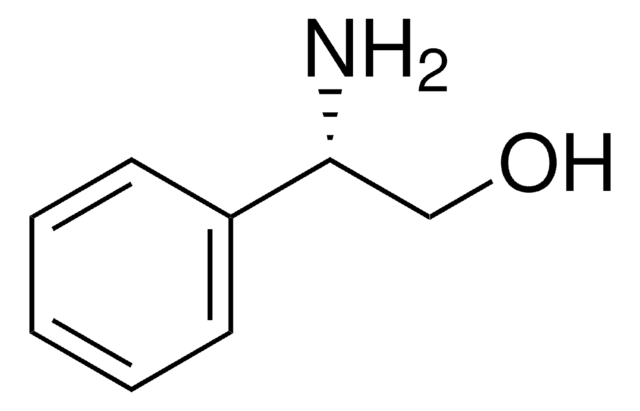
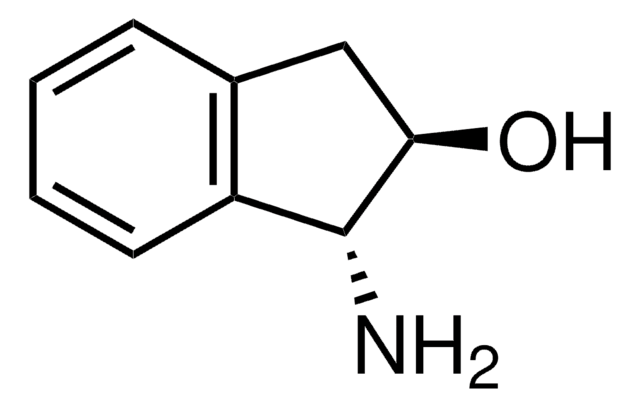
(1S,2R)-(−)-cis-1-Amino-2-indanol
99%
Synonym(s):
(1S,2R)-(−)-cis-1-Amino-2-hydroxyindane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H11NO
CAS Number:
Molecular Weight:
149.19
Beilstein:
4292559
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
optical purity
ee: 99% (GLC)
mp
118-121 °C (lit.)
SMILES string
N[C@@H]1[C@H](O)Cc2ccccc12
InChI
1S/C9H11NO/c10-9-7-4-2-1-3-6(7)5-8(9)11/h1-4,8-9,11H,5,10H2/t8-,9+/m1/s1
InChI key
LOPKSXMQWBYUOI-BDAKNGLRSA-N
Looking for similar products? Visit Product Comparison Guide
General description
(1S,2R)-(-)-cis-1-Amino-2-indanol is a main constituent of indinavir, a potent HIV (human immunodeficiency virus) protease inhibitor.
Application
1S,2R)-(-)-cis-1-Amino-2-indanol may be used to prepare:
- (-)-1,2,5,6-Tetrahydropyridine by reacting with methyl (E,E)-4-oxo-2-[(2,6,6-trimethylcyclohex-1-enyl)vinyl}but-2-enoate.
- Oxazaborolidine catalysts, which can catalyze the asymmetric reduction of aromatic ketones with high enantioselectivity.
- (RS,1S,2R)-(-)-2,4,6-Trimethylbenzenesulfinic acid 1-(2,4,6-trimethylbenzenesulfonylamino)indan-2-yl ester.
Used to prepare a mannitol-based scaffold in the study of Plasmepsin II inhibition. Aspartic proteases such as plasmepsins I and II are of interest as targets for new, potential anti-malarials.
Physical properties
Useful chiral ligand for asymmetric synthesis.
Legal Information
Sold under license from Sterling Pharma Solutions Limited.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karolina Ersmark et al.
Bioorganic & medicinal chemistry, 11(17), 3723-3733 (2003-08-07)
A series of C(2)-symmetric compounds with a mannitol-based scaffold has been investigated, both theoretically and experimentally, as Plm II inhibitors. Four different stereoisomers with either benzyloxy or allyloxy P1/P1' side chains were studied. Computational ranking of the binding affinities of
Improved asymmetric synthesis of aziridine 2-phosphonates using (S)-(+)-2, 4, 6-trimethylphenylsulfinamide.
Davis FA, et al.
The Journal of Organic Chemistry, 68(18), 6894-6898 (2003)
Chemoenzymatic synthesis of (1S, 2R)-1-amino-2-indanol, a key intermediate of HIV protease inhibitor, indinavir.
Demir AS, et al.
Journal of Molecular Catalysis. B, Enzymatic, 9(4), 157-161 (2000)
Highly stereoselective asymmetric 6p-azaelectrocyclization utilizing the novel 7-alkyl substituted cis-1-amino-2-indanols: Formal synthesis of 20-epiuleine.
Tanaka K and Katsumura S.
Journal of the American Chemical Society, 124(33), 9660-9661 (2002)
Cis-1-amino-2-indanol in asymmetric synthesis. Part I. A practical catalyst system for the enantioselective borane reduction of aromatic ketones.
Hong Y, et al.
Tetrahedron Letters, 35(36), 6631-6634 (1994)
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service