All Photos(1)
About This Item
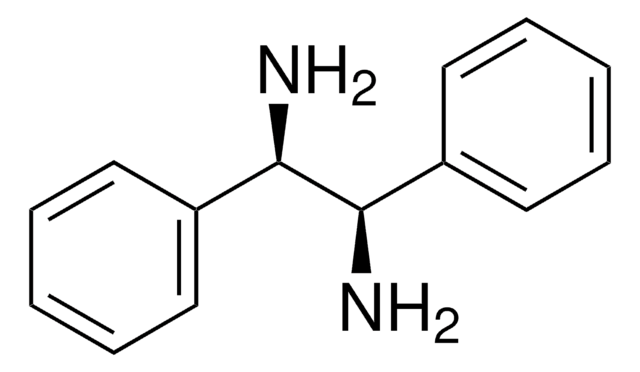
Linear Formula:
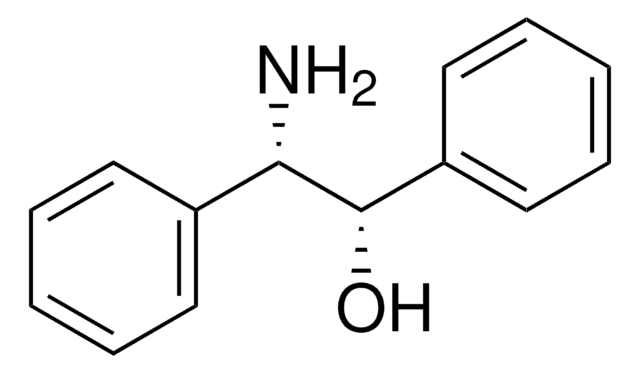
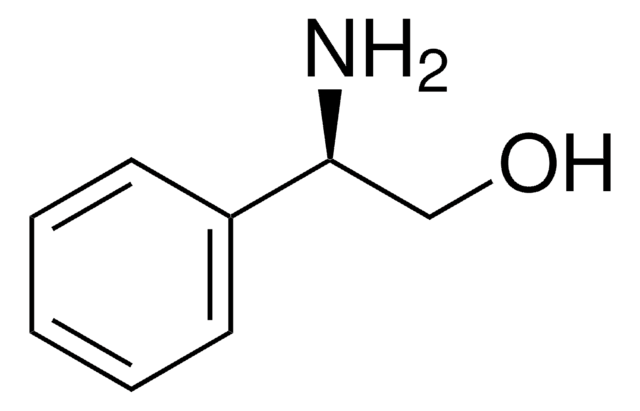
C6H5CH(NH2)CH(C6H5)OH
CAS Number:
Molecular Weight:
213.28
Beilstein:
2806218
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
optical activity
[α]25/D −7.0°, c = 0.6 in ethanol
mp
142-144 °C (lit.)
SMILES string
N[C@H]([C@H](O)c1ccccc1)c2ccccc2
InChI
1S/C14H15NO/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-14,16H,15H2/t13-,14+/m0/s1
InChI key
GEJJWYZZKKKSEV-UONOGXRCSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Chiral auxiliary used for Pd(II)-assisted chiral tandem alkylation and carbonylative coupling reactions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J.J. Masters et al.
The Journal of Organic Chemistry, 58, 4547-4547 (1993)
J.J. Masters et al.
The Journal of Organic Chemistry, 56, 5666-5666 (1991)
Michael L Berger et al.
Bioorganic & medicinal chemistry, 17(9), 3456-3462 (2009-04-07)
We resolved 1,2-diphenylethylamine (DPEA) into its (S)- and (R)-enantiomer and used them as precursors for synthesis of (S)- and (R)-1-(1,2-diphenylethyl)piperidine, flexible homeomorphs of the NMDA channel blocker MK-801. We also describe the synthesis of the dicyclohexyl analogues of DPEA. These
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service