391662
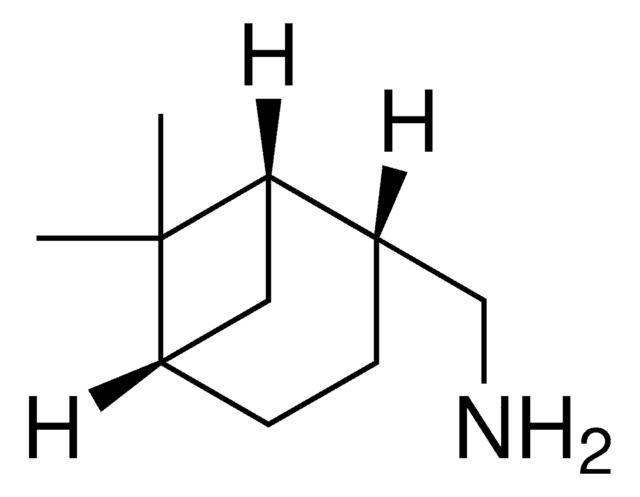
(1S,2S,3S,5R)-(+)-Isopinocampheylamine
95%
Synonym(s):
(+)-Isopinocampheylamine, (1S,2S,3S,5R)-3-Pinanamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H19N
CAS Number:
Molecular Weight:
153.26
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
optical activity
[α]22/D +44°, neat
refractive index
n20/D 1.481 (lit.)
bp
90 °C/18 mmHg (lit.)
density
0.909 g/mL at 25 °C (lit.)
SMILES string
C[C@@H]1[C@@H](N)C[C@H]2C[C@@H]1C2(C)C
InChI
1S/C10H19N/c1-6-8-4-7(5-9(6)11)10(8,2)3/h6-9H,4-5,11H2,1-3H3/t6-,7+,8-,9-/m0/s1
InChI key
VPTSZLVPZCTAHZ-KZVJFYERSA-N
Application
(1S,2S,3S,5R)-(+)-isopinocampheylamine is a primary bicyclic amine with potent M2 ion channel inhibitor ability similar to that of amantadine, making it a promising candidate for developing anti-influenza agents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
161.6 °F - closed cup
Flash Point(C)
72 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Discovery of highly potent agents against influenza A virus.
Zhao X, et al.
European Journal of Medicinal Chemistry, 46(1), 52-57 (2011)
Identification of hits as matrix-2 protein inhibitors through the focused screening of a small primary amine library.
Hu W, et al.
Journal of Medicinal Chemistry, 53(9), 3831-3834 (2010)
New strategy for high throughput screening of anti-influenza virus M2 ion channel inhibitors.
Li C, et al.
Current Pharmaceutical Design, 19(28), 5146-5155 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![(+)-Bis[(R)-1-phenylethyl]amine 99%](/deepweb/assets/sigmaaldrich/product/structures/188/828/177cd49c-056f-47d3-976c-c8cdcd5f62c5/640/177cd49c-056f-47d3-976c-c8cdcd5f62c5.png)