389374
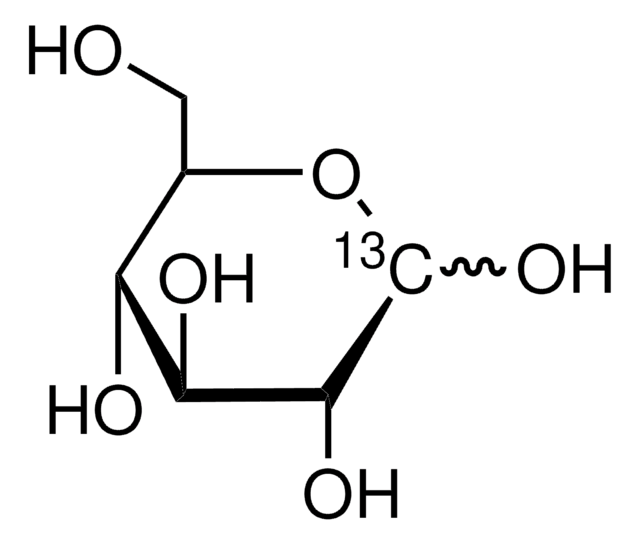
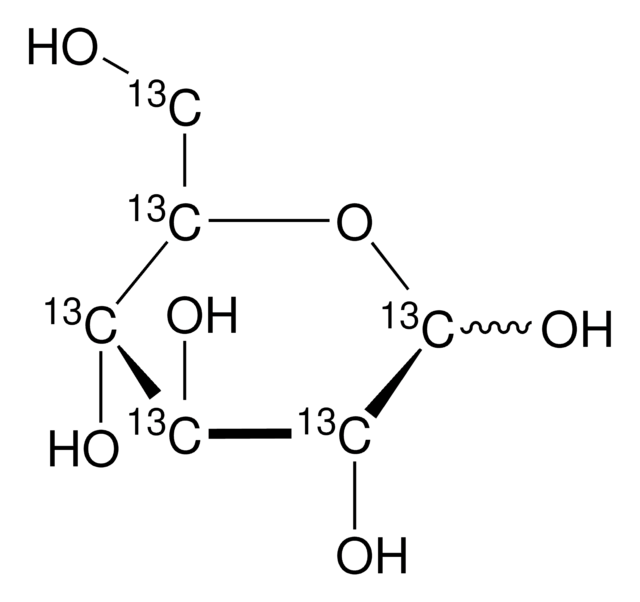
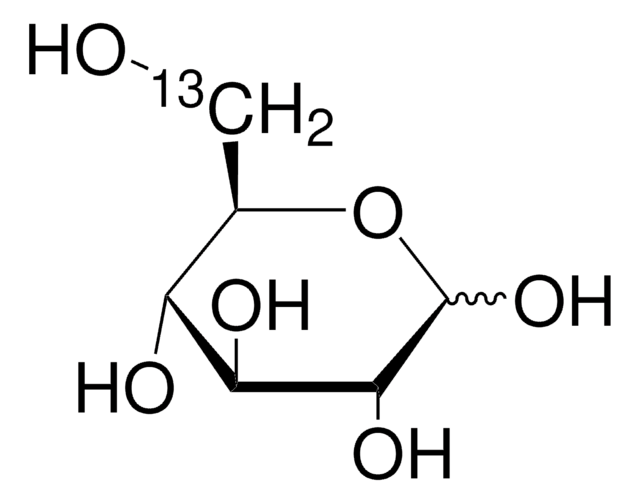
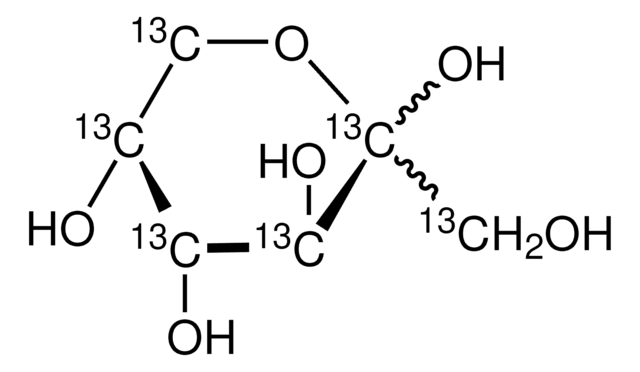
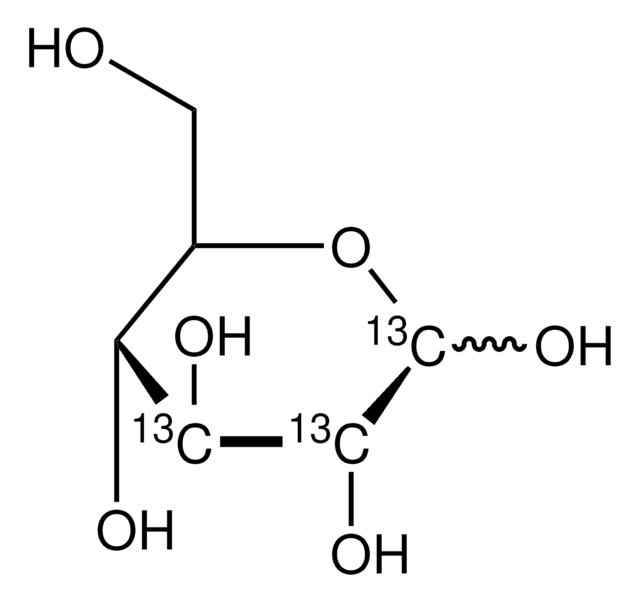
D-Glucose-13C6
≥99 atom % 13C, ≥99% (CP)
Synonym(s):
Labeled Glucose, D-Glucose-ul-13C, Dextrose-13C6
About This Item
Recommended Products
isotopic purity
≥99 atom % 13C
Quality Level
Assay
≥99% (CP)
form
powder
optical activity
[α]25/D +52.0°, c = 2 in H2O (trace NH4OH)
technique(s)
bio NMR: suitable
protein expression: suitable
protein purification: suitable
mp
150-152 °C (lit.)
mass shift
M+6
SMILES string
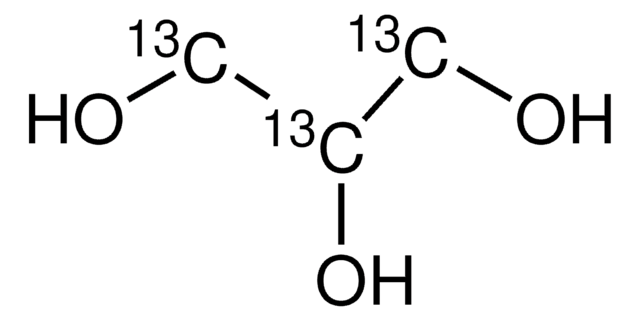
O[13CH2][13C@H]1O[13C@H](O)[13C@H](O)[13C@@H](O)[13C@@H]1O
InChI
1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6?/m1/s1/i1+1,2+1,3+1,4+1,5+1,6+1
InChI key
WQZGKKKJIJFFOK-NYKRWLDMSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- for de novo fatty acid (FA) synthesis
- as a component in tracing media for the analysis of stable isotope tracer
- in minimal media for recombinant protein production
- for cell suspension
Biochem/physiol Actions
Packaging
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Our company presents an informative page regarding the use of ISOTEC Stable Isotopes to Amplify Recombinant Protein Expression with ISOGRO.
Utilizing ISOGRO® Supplementation of M9 Minimal Media to Enhance Recombinant Protein Expression.
Solid-state NMR on Larger Biomolecules; Sigma-Aldrich.com
Learn about monosaccharide biosynthesis and the metabolism of monosaccharides. A unit of a carbohydrate and the simplest form of a sugar, a monosaccharide cannot be hydrolyzed into a simpler compound.
Related Content
Protein structure provides valuable information that can be used to infer protein function. The study of protein structure and mapping of protein interactions, expression levels, and location enables the identification of disease biomarkers and potential drug targets for therapeutic treatment.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service