373605
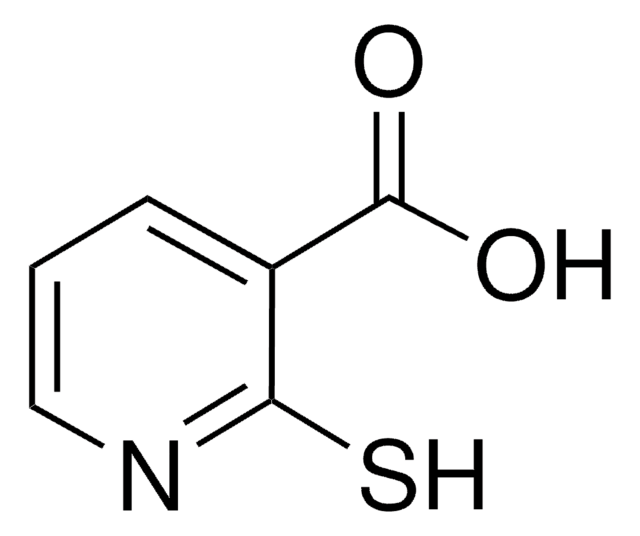
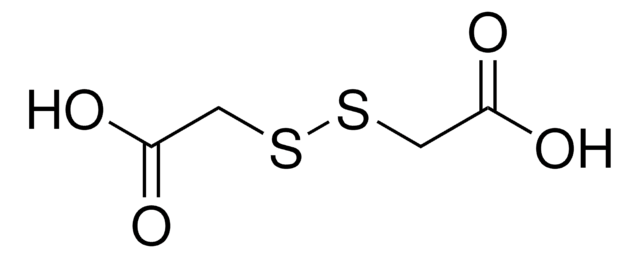
6,6′-Dithiodinicotinic acid
technical grade, 85%
Synonym(s):
Bis[3-Carboxypyridine] 6,6′-disulfide, Bis[3-carboxypyridine] 6,6′-disulfide
About This Item
Recommended Products
grade
technical grade
Assay
85%
form
solid
mp
263-265 °C (lit.)
SMILES string
OC(=O)c1ccc(SSc2ccc(cn2)C(O)=O)nc1
InChI
1S/C12H8N2O4S2/c15-11(16)7-1-3-9(13-5-7)19-20-10-4-2-8(6-14-10)12(17)18/h1-6H,(H,15,16)(H,17,18)
InChI key
GSASOFRDSIKDSN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- specific thiol blocker to investigate the radioprotective effect of mercaptoethylamine on the thiol level of the outer cell membrane of the Ehrlich ascites tumor cells
- modifier to investigate the polar microenvironment around the reactive Cys283 of rabbit muscle creatine kinase
- dipyridyl-dithio substrate to evaluate the protein disulfide-thiol interchange activity of the auxin stimulated NADH: protein disulfide reductase (NADH oxidase) of soybean plasma membranes
- water-soluble reagent in a study for introducing, in buffered saline, a reactive sulfhydryl group on water-soluble molecules bearing an alkyl-amino group
- chromogen for sulfhydryl groups in the Ellman method for cholinesterase determinations
- spectrophotometric determination of thiols and of total glutathione in human blood
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service