365858
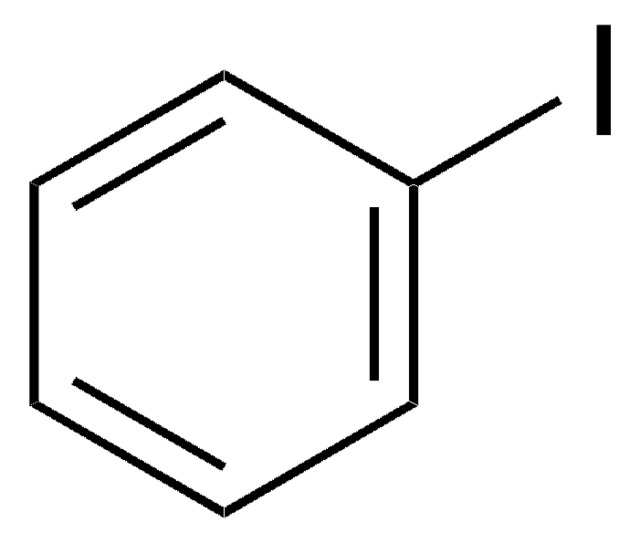
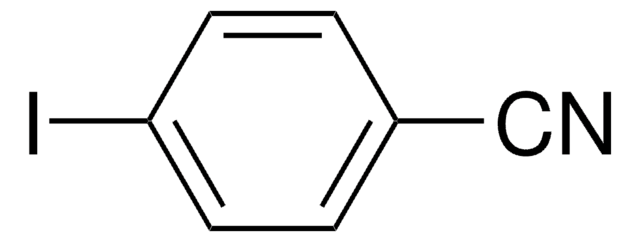
4-Iodobenzotrifluoride
97%
Synonym(s):
4-Iodo-α,α,α-trifluorotoluene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H4CF3
CAS Number:
Molecular Weight:
272.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.516 (lit.)
bp
185-186 °C/745 mmHg (lit.)
mp
−8.33 °C (lit.)
density
1.851 g/mL at 25 °C (lit.)
SMILES string
FC(F)(F)c1ccc(I)cc1
InChI
1S/C7H4F3I/c8-7(9,10)5-1-3-6(11)4-2-5/h1-4H
InChI key
SKGRFPGOGCHDPC-UHFFFAOYSA-N
Related Categories
General description
4-Iodobenzotrifluoride undergoes aminocarbonylation in DMF using phosphoryl chloride to give N,N-dimethyl-(4-trifluoromethyl)benzamide. Mechanism of the copper-free Sonogashira cross-coupling reaction of 4-iodobenzotrifluoride with differently para-substituted phenylacetylenes has been investigated.
Application
4-Iodobenzotrifluoride may be employed as substrate with electron-deficient aromatic ring, during Mizoroki-Heck reaction with acrylic acid, to afford 4-trifluoromethylcinnnamic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
156.2 °F - closed cup
Flash Point(C)
69 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kazushi Hosoi et al.
Organic letters, 4(17), 2849-2851 (2002-08-17)
[reaction: see text] Palladium-catalyzed coupling reaction of N,N-dimethylformamide with aryl or alkenyl halides successfully proceeded in the presence of phosphoryl chloride to afford the corresponding tertiary amides in good yields.
Two competing mechanisms for the copper-free Sonogashira cross-coupling reaction.
Ljungdahl T, et al.
Organometallics, 27(11), 2490-2498 (2008)
Atsushi Ohtaka et al.
Molecules (Basel, Switzerland), 16(11), 9067-9076 (2011-10-29)
Linear polystyrene-stabilized PdO nanoparticles (PS-PdONPs) were prepared by thermal decomposition of Pd(OAc)(2) in the presence of polystyrene. X-ray diffraction (XRD) and transmission electron microscopy (TEM) indicated the production of PdO nanoparticles. The loading of palladium was determined by inductively coupled
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service