359475
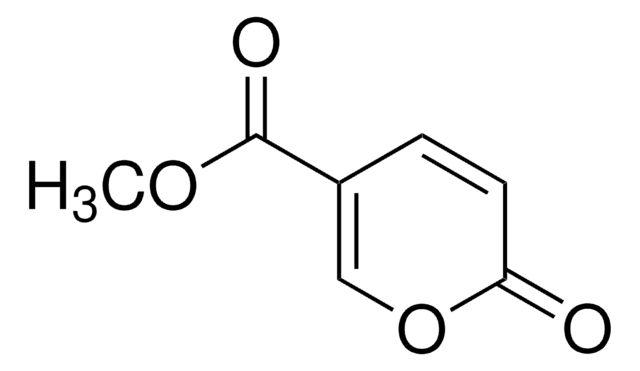
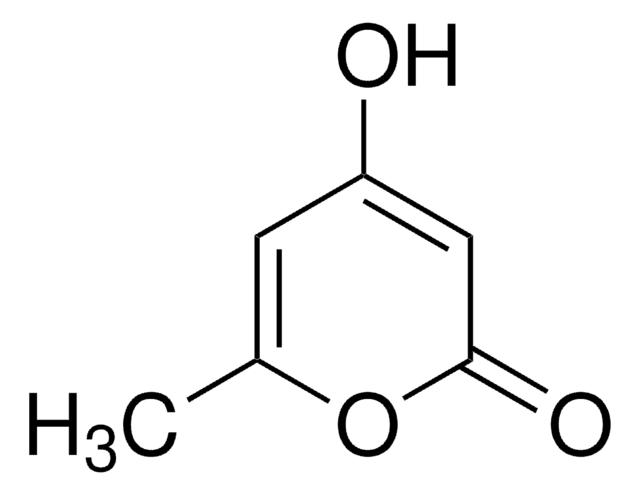
Methyl 2-oxo-2H-pyran-3-carboxylate
98%
Synonym(s):
Methyl 2-pyrone-3-carboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H6O4
CAS Number:
Molecular Weight:
154.12
Beilstein:
1424749
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
bp
146-148 °C/0.75 mmHg (lit.)
mp
75-77 °C (lit.)
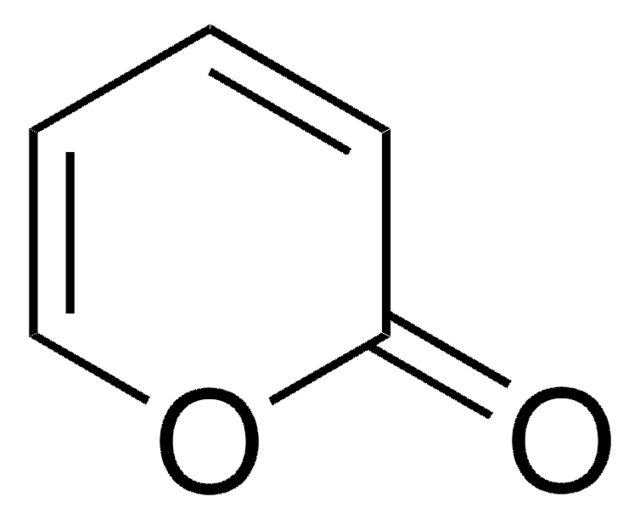
SMILES string
COC(=O)C1=CC=COC1=O
InChI
1S/C7H6O4/c1-10-6(8)5-3-2-4-11-7(5)9/h2-4H,1H3
InChI key
GJVZWOMUTYNUCE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Methyl 2-oxo-2H-pyran-3-carboxylate is a cyclic α,β -unsaturated ketone. It activates caspases-3, -8 and -9 weakly in HL-60 cells.
Application
Methyl 2-oxo-2H-pyran-3-carboxylate is suitable reagent used to investigate the series of simple α,β-unsaturated carbonyl compounds for their cytotoxic profiles against oral human normal and tumor cells.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Risa Takeuchi et al.
Anticancer research, 26(5A), 3343-3348 (2006-11-11)
As previously suggested, codeinone (oxidation product of codeine) induces non-apoptotic cell death, characterized by marginal caspase activation and the lack of DNA fragmentation in HL-60 human promyelocytic leukemia cells, which was inhibited by N-acetyl-L-cysteine. Whether, morphinone, an oxidative metabolite of
Tohru Nakayachi et al.
Anticancer research, 24(2B), 737-742 (2004-05-27)
A series of simple alpha, beta-unsaturated carbonyl compounds (1-26) was characterized for their cytotoxic profiles against oral human normal and tumor cells. Several cycloalkenones showed potent cytotoxic activities against human oral squamous cell carcinoma HSC-2 cell line. Among them, 4,4-dimethyl-2-cyclopenten-1-one
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service