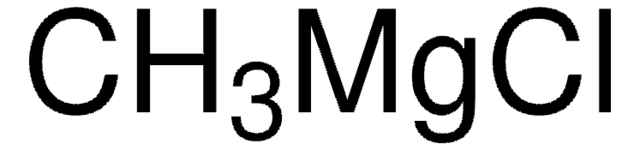282235
Methylmagnesium bromide solution
1.4 M in THF: toluene (1:3)
Synonym(s):
Bromomethylmagnesium
About This Item
Recommended Products
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
1.4 M in THF: toluene (1:3)
density
1.018 g/mL at 25 °C
SMILES string
C[Mg]Br
InChI
1S/CH3.BrH.Mg/h1H3;1H;/q;;+1/p-1
InChI key
AVFUHBJCUUTGCD-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
This Item | 1535755 | 1535722 | 1372061 |
|---|---|---|---|
| manufacturer/tradename USP | manufacturer/tradename USP | manufacturer/tradename USP | manufacturer/tradename USP |
| format neat | format neat | format neat | format neat |
| application(s) pharmaceutical (small molecule) | application(s) pharmaceutical (small molecule) | application(s) pharmaceutical (small molecule) | application(s) pharmaceutical (small molecule) |
| API family defatted powdered soy | API family phosphatidylcholine | API family phosphatidic acid | API family lysophosphatidylcholine |
| storage temp. -10 to -25°C | storage temp. −20°C | storage temp. −20°C | storage temp. −20°C |
Application
Signal Word
Danger
Hazard Statements
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1B - STOT RE 2 - STOT SE 3 - Water-react 1
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service