171565
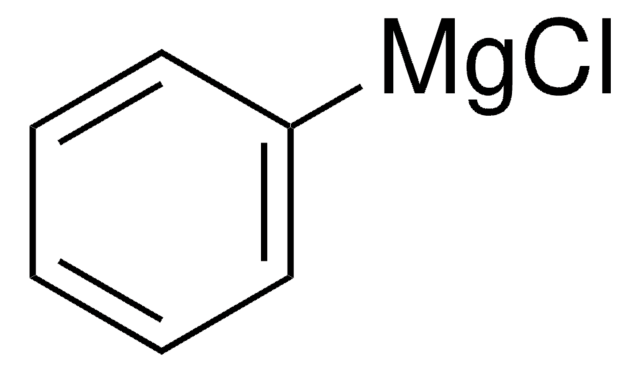
Phenylmagnesium bromide solution
3.0 M in diethyl ether
Synonym(s):
Bromomagnesiobenzene, Bromophenylmagnesium
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5MgBr
CAS Number:
Molecular Weight:
181.31
Beilstein:
3588849
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
3.0 M in diethyl ether
density
1.134 g/mL at 25 °C
SMILES string
Br[Mg]c1ccccc1
InChI
1S/C6H5.BrH.Mg/c1-2-4-6-5-3-1;;/h1-5H;1H;/q;;+1/p-1
InChI key
ANRQGKOBLBYXFM-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Phenylmagnesium bromide solution contains 3M phenylmagnesium bromide in diethyl ether. It can act as a strong acid and Lewis acid. It can undergo addition reaction with many unsaturated functional groups. The phenyl group can displace halide from other organic compounds. Phenylmagnesium bromide is a Grignard reagent. Reaction of β-cyclohexanedione (dihydroresorcinol) with phenylmagnesium bromide has been investigated.
Application
Phenylmagnesium bromide was used for the synthesis of end-functionalized regioregular poly(3-alkylthiophene)s. It was also used for the monoalkylation of aliphatic primary amine to generate secondary amines by the Grignard reaction of 1-[(alkylamino) methyl] benzotriazoles.
It may be used for synthesis of the following:
It may be used for synthesis of the following:
- 1,3,3-trimethyl-6-phenyl-2-oxabicyclo[2.2.2]octan-6-ol
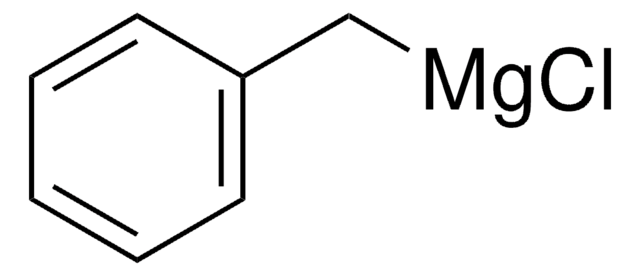
- 6-benzyl-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol
- (3-(2-Dithiobenzoatepropionyl)propyl)dimethylmethoxysilane, reversible addition-fragmentation chain transfer polymerization (RAFT)-silane agent
- series of o-substituted benzophenones
Packaging
Other Notes
Storage below 25°C may cause formation of crystalline magnesium salts. Moving container to a warm location and occasional gentle swirling should redissolve the solid
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-40.0 °F - closed cup
Flash Point(C)
-40 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of O-substituted benzophenones by Grignard reaction of 3-substituted isocoumarins.
Manivel P, et al.
Journal of the Chilean Chemical Society, 53(3), 1609-1610 (2008)
The reaction of beta-cyclohexanedione (dihydroresorcinol) and its ethyl enol ether with phenylmagnesium bromide.
G F WOODS et al.
Journal of the American Chemical Society, 70(6), 2174-2177 (1948-06-01)
Synthesis of well-defined polymer brushes grafted onto silica nanoparticles via surface reversible addition-fragmentation chain transfer polymerization.
Li C and Benicewicz BC.
Macromolecules, 38(14), 5929-5936 (2005)
S Schenone et al.
Farmaco (Societa chimica italiana : 1989), 45(12), 1309-1325 (1990-12-01)
The synthesis of 1,3,3-trimethyl-6-phenyl-2-oxabicyclo[2.2.2]octan-6-ol 2 and 6-benzyl-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol 3 starting from (+)-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-one and phenylmagnesium bromide or benzylmagnesium chloride, respectively, is described. Alcohols 2 and 3 gave a series of omega-dialkylaminoalkyl ethers 4 by reaction as sodium salts with omega-chloroalkyldialkylamines in toluene
Phenylmagnesium Bromide.
Richey HG, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service