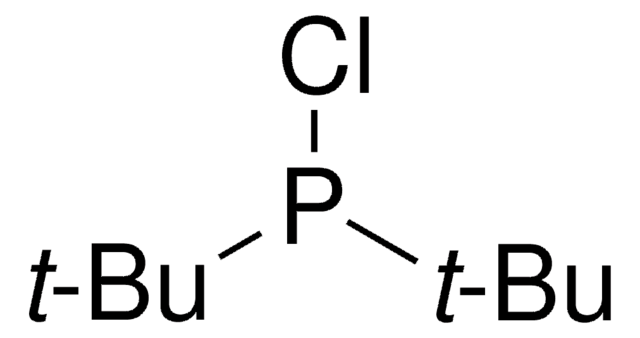
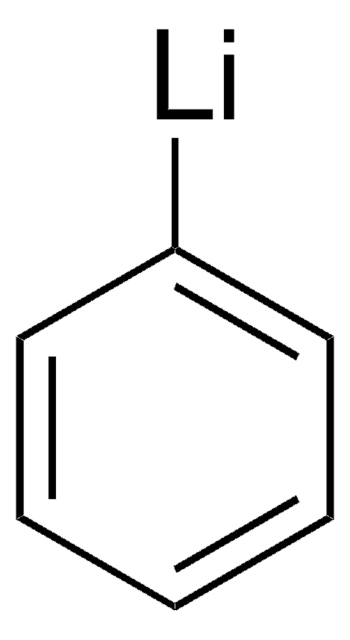
593230
Phenyllithium solution
1.9 M in dibutyl ether
Synonym(s):
PhLi
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H5Li
CAS Number:
Molecular Weight:
84.04
Beilstein:
506502
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
concentration
1.9 M in dibutyl ether
density
0.835 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
[Li]c1ccccc1
InChI
1S/C6H5.Li/c1-2-4-6-5-3-1;/h1-5H;
InChI key
NHKJPPKXDNZFBJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Phenyllithium solution can be used to synthesize:
- 1-Acetyl-1′-diphenylphosphinoferrocene from ferrocenophane.
- Ethyl 2-acetylimino-1-benzyl-6-bromo-4-phenyl-1,4-dihydroquinoline-3-carboxylate from ethyl 1-benzyl-6-bromo-2-imino-1,2-dihydroquinoline-3-carboxylate.
- Ethyl 2-amino-1-benzoyl-6-bromo-4-phenyl-1,4-dihydroquinoline-3-carboxylate from (E)-ethyl 2-(acetylimino)-1-benzoyl-6-bromo-1,2-dihydroquinoline-3-carboxylate.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Flash Point(F)
77.0 °F - closed cup
Flash Point(C)
25 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The first synthesis of 2-amino-1, 4-dihydroquinolines.
Viault G, et al.
Tetrahedron, 65(49), 10149-10154 (2009)
The synthesis of ?-N, N-dimethyl-1?-diphenylphosphinoferrocenylethylamine and related ligands.
Butler I R, et al.
Canadian Journal of Chemistry, 61(1), 147-153 (1983)
Luka A Wright et al.
Dalton transactions (Cambridge, England : 2003), 44(16), 7230-7241 (2015-03-20)
The 2-(2′-aniline)-6-imine-pyridines, 2-(C6H4-2′-NH2)-6-(CMe=NAr)C5H3N (Ar = 4-i-PrC6H4 (HL1a), 2,6-i-Pr2C6H3 (HL1b)), have been synthesised via sequential Stille cross-coupling, deprotection and condensation steps from 6-tributylstannyl-2-(2-methyl-1,3-dioxolan-2-yl)pyridine and 2-bromonitrobenzene. The palladium(II) acetate N,N,N-pincer complexes, [{2-(C6H4-2′-NH)-6-(CMe=NAr)C5H3N}Pd(OAc)] (Ar = 4-i-PrC6H4 (1a), 2,6-i-Pr2C6H3 (1b)), can be prepared by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service