245933
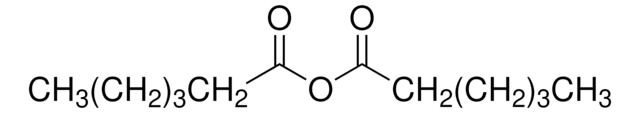
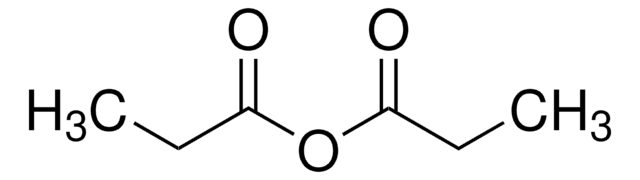
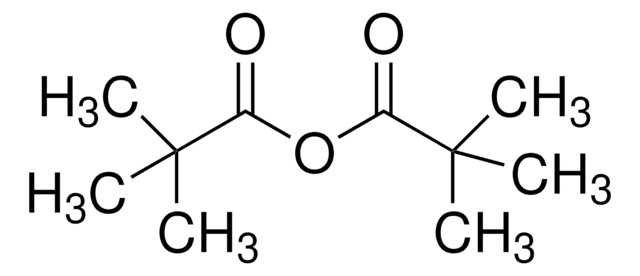
Valeric anhydride
97%
Synonym(s):
Pentanoic anhydride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
[CH3(CH2)3CO]2O
CAS Number:
Molecular Weight:
186.25
Beilstein:
1770130
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.421 (lit.)
bp
228-230 °C (lit.)
mp
−56 °C (lit.)
density
0.944 g/mL at 20 °C (lit.)
SMILES string
CCCCC(=O)OC(=O)CCCC
InChI
1S/C10H18O3/c1-3-5-7-9(11)13-10(12)8-6-4-2/h3-8H2,1-2H3
InChI key
DUCKXCGALKOSJF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Valeric anhydride can be used as a reactant to synthesize:
- Alkyl 9-nitrocamptothecin esters by the esterification reaction.
- Modified bismuth metal-organic frameworks (Bi-MOFs).
- O-acylated chitosan nanofibers (CSNFs) for potential usage in biomaterials and food packaging.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
210.2 °F - closed cup
Flash Point(C)
99 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
O-acylation of chitosan nanofibers by short-chain and long-chain fatty acids
Zhang Z, et al.
Carbohydrate Polymers, 177, 203-209 (2017)
Zhi-Song Cao et al.
Acta pharmacologica Sinica, 24(2), 109-119 (2003-01-28)
To study the structure-activity relationship of alkyl 9-nitrocamptothecin esters. Two alkyl 9-nitrocamptothecin (9NC) esters 5g and 5h were prepared by esterification reactions of 9NC with valeric anhydride and heptanoic anhydride, respectively. Eight 9NC esters 5a-5h were tested for cytotoxicity against
Synthesis, functionalisation and post-synthetic modification of bismuth metal-organic frameworks
Koppen M, et al.
Dalton Transactions, 46(26), 8658-8663 (2017)
Karolina Skołucka-Szary et al.
Materials science & engineering. C, Materials for biological applications, 55, 50-60 (2015-06-29)
In this article, the synthesis of novel biopolymer, chitin dipentanoate (Di-O-Valeryl Chitin, DVCH) has been described. DVCH is a chitin derivative esterified with two valeryl groups at positions 3 and 6 of the N-acetylglucosamine units and it is soluble in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service