220027
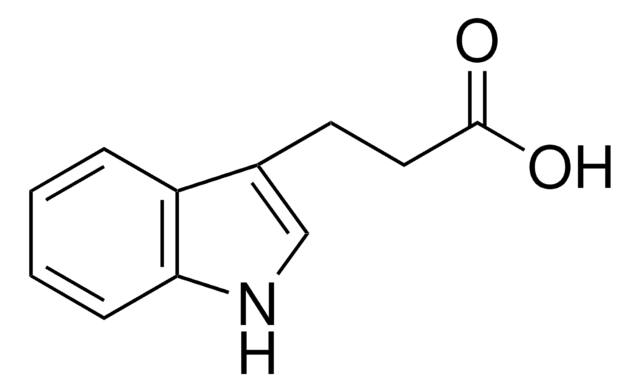
3-Indolepropionic acid
ReagentPlus®, 99%
Synonym(s):
3-(3-Indolyl)propanoic acid, IPA, Indole-3-propionic acid, NSC 3252, NSC 47831
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H11NO2
CAS Number:
Molecular Weight:
189.21
Beilstein:
147733
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
form
powder
mp
134-135 °C (lit.)
solubility
ethanol: soluble 50 mg/mL, clear, yellow to orange
SMILES string
OC(=O)CCc1c[nH]c2ccccc12
InChI
1S/C11H11NO2/c13-11(14)6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6H2,(H,13,14)
InChI key
GOLXRNDWAUTYKT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Indolepropionic acid is an effective inhibitor of aggregation of misfolded β-amyloid protein (Abeta). Three-component one-pot procedure has been reported to assemble 3-indolepropionic acids.
Application
Reactant for preparation of:
- Fluorescent analogues of strigolactones
- Anti-tumor agents
- Melanocortin receptors ligands
- Immunosuppressive agents
- Iinhibitors of hepatitis C virus
- Histamine H4 receptor agonists
- NR2B/NMDA receptor antagonists
- CB1 Antagonist for the treatment of obesity
- Antibacterial agents
- Inhibitor of TGF-β receptor binding
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mauro F A Adamo et al.
Organic letters, 9(2), 303-305 (2007-01-16)
A three-component one-pot procedure (3-MC) was developed to assemble 3-indolepropionic acids from commercially available materials. This new methodology affords the title compounds in high yields and without the use of chromatography. [reaction: see text].
Xun Cheng et al.
Analytical chemistry, 77(21), 7012-7015 (2005-11-01)
Alzheimer's disease is the most common cause of the loss of cognitive function among the elderly, and the aggregation and deposition of misfolded beta-amyloid protein (Abeta) contribute to this progressive central nervous system decline. Therefore, compounds that inhibit or even
Andrew W Woodward et al.
Plant physiology, 144(2), 976-987 (2007-04-24)
The ubiquitin-like protein RELATED TO UBIQUITIN (RUB) is conjugated to CULLIN (CUL) proteins to modulate the activity of Skp1-Cullin-F-box (SCF) ubiquitylation complexes. RUB conjugation to specific target proteins is necessary for the development of many organisms, including Arabidopsis (Arabidopsis thaliana).
B Poeggeler et al.
Brain research, 815(2), 382-388 (1999-01-08)
The hydroxyl radical scavenging activity of indole-3-propionate was evaluated by kinetic competition studies with the hydroxyl radical trapping reagent 2,2'-azino-bis-(3-ethyl-benz-thiazoline-6-sulfonic acid) (ABTS) and by measuring hydroxyl radical-initiated lipid peroxidation in the rat striatum. Using ABTS, the indole was shown to
K L Borden et al.
European journal of biochemistry, 202(2), 459-470 (1991-12-05)
The antirepressor indole 3-propanoate has been shown by X-ray crystallography to bind in a different orientation compared with the natural corepressor for the tryp repressor, L-tryptophan (Lawson, C.L. & Sigler, P. B. (1988) Nature 333, 869-871). This suggests a simple
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service