I7017
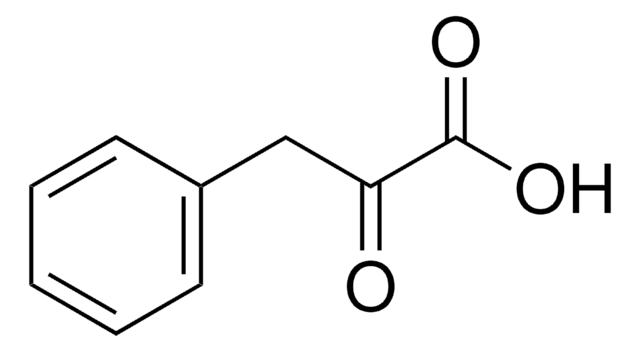
Indole-3-pyruvic acid
≥97%
Synonym(s):
3-(3-Indolyl)-2-oxopropanoic acid, 3-Indolylpyruvic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C11H9NO3
CAS Number:
Molecular Weight:
203.19
Beilstein:
172966
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
color
light yellow
mp
215 °C (dec.) (lit.)
storage temp.
−20°C
SMILES string
OC(=O)C(=O)Cc1c[nH]c2ccccc12
InChI
1S/C11H9NO3/c13-10(11(14)15)5-7-6-12-9-4-2-1-3-8(7)9/h1-4,6,12H,5H2,(H,14,15)
InChI key
RSTKLPZEZYGQPY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Indole-3-pyruvic acid can be used:
- As a precursor for the synthesis of chromopyrrolic acid by a heme-containing enzyme.
- As a reactant in the Biginelli-like scaffold syntheses.
Linkage
α-Keto analogue of tryptophan
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cyclic ketones and substituted α-keto acids as alternative substrates for novel Biginelli-like scaffold syntheses
Abelman, Matthew M, et al.
Tetrahedron Letters, 44(24), 4559-4562 (2003)
L Bacciottini et al.
Pharmacological research communications, 19(11), 803-817 (1987-11-01)
The effects of acute or repeated administration of indole-pyruvic acid (IPA), a keto-analogue of tryptophan (TRP), were studied in various brain areas of rats by measuring the changes of 5-hydroxytryptamine (5-HT) and of norepinephrine (NE) content and metabolism. The analgesic
Direct formation of chromopyrrolic acid from indole-3-pyruvic acid by StaD, a novel hemoprotein in indolocarbazole biosynthesis
Asamizu, S, et al.
Tetrahedron Letters, 47(4), 473-475 (2006)
Thomas Rauhut et al.
Phytochemistry, 70(15-16), 1638-1644 (2009-06-16)
Structurally related secondary products are rather rarely shared by organisms from different kingdoms. Consequently, the evolution of biosynthetic pathways of defence metabolites between distantly related organisms has not been broadly investigated. Thiazolylindoles are found in Arabidopsis thaliana, as the phytoalexin
Anna N Stepanova et al.
Cell, 133(1), 177-191 (2008-04-09)
Plants have evolved a tremendous ability to respond to environmental changes by adapting their growth and development. The interaction between hormonal and developmental signals is a critical mechanism in the generation of this enormous plasticity. A good example is the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service