157422
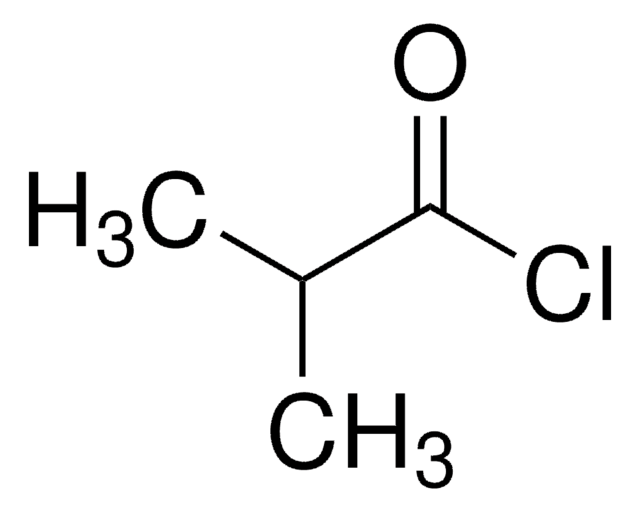
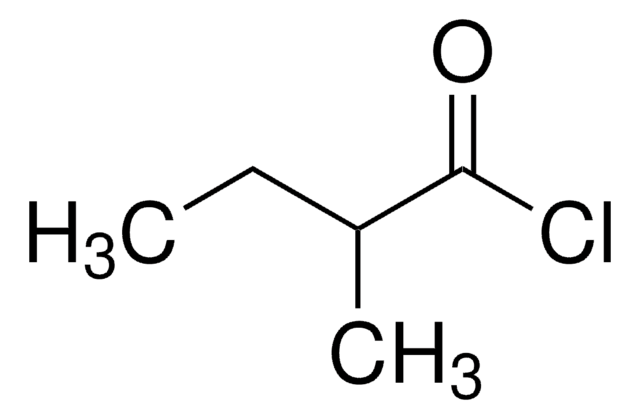
Isovaleryl chloride
98%
Synonym(s):
3-Methylbutyryl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH2COCl
CAS Number:
Molecular Weight:
120.58
Beilstein:
741910
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.416 (lit.)
bp
115-117 °C (lit.)
density
0.989 g/mL at 25 °C (lit.)
SMILES string
CC(C)CC(Cl)=O
InChI
1S/C5H9ClO/c1-4(2)3-5(6)7/h4H,3H2,1-2H3
InChI key
ISULZYQDGYXDFW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Isovaleryl chloride was used in the synthesis of:
- furanodictines A and B
- tetrapeptide amide, S-benzyl-L-cysteinyl-L-prolyl-L-leucylglycinamide
- (+)-blastmycinone
- (R)- and (S)-2-methyl-4-octanol, aggregation pheromone of some sugarcane weevils
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
87.8 °F - closed cup
Flash Point(C)
31 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enantioselective synthesis of (R)-and (S)-2-methyl-4-octanol, the male-produced aggregation pheromone of Curculionidae species.
Baraldi PT, et al.
Tetrahedron Asymmetry, 13(6), 621-624 (2002)
The Synthesis of the Tetrapeptide Amide S-Benzyl-L-cysteinyl-L-prolyl-L-leucylglycinamide
Ressler Cand Vincent du Vigneaud.
Journal of the American Chemical Society, 76(12), 3107-3109 (1954)
Makoto Ogata et al.
Carbohydrate research, 345(2), 230-234 (2009-12-08)
A novel synthesis of furanodictines A [2-acetamido-3,6-anhydro-2-deoxy-5-O-isovaleryl-D-glucofuranose (1)] and B [2-acetamido-3,6-anhydro-2-deoxy-5-O-isovaleryl-D-mannofuranose (2)] is described starting from 2-acetamido-2-deoxy-D-glucose (GlcNAc). The synthetic protocol is based on deriving the epimeric bicyclic 3,6-anhydro sugars [2-acetamido-3,6-anhydro-2-deoxy-D-glucofuranose (4) and 2-acetamido-3,6-anhydro-2-deoxy-D-mannofuranose (5)] from GlcNAc. Reaction with borate
Organoaluminium induced ring-opening of epoxypyranosides. V. Formal total synthesis of antimycin A3 and synthesis of (+)-blastmycinone.
Inghardt T and Frejd T.
Tetrahedron, 47(32), 6483-6492 (1991)
Jessica L Wojtaszek et al.
Cell, 178(1), 152-159 (2019-06-11)
Intrinsic and acquired drug resistance and induction of secondary malignancies limit successful chemotherapy. Because mutagenic translesion synthesis (TLS) contributes to chemoresistance as well as treatment-induced mutations, targeting TLS is an attractive avenue for improving chemotherapeutics. However, development of small molecules with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service