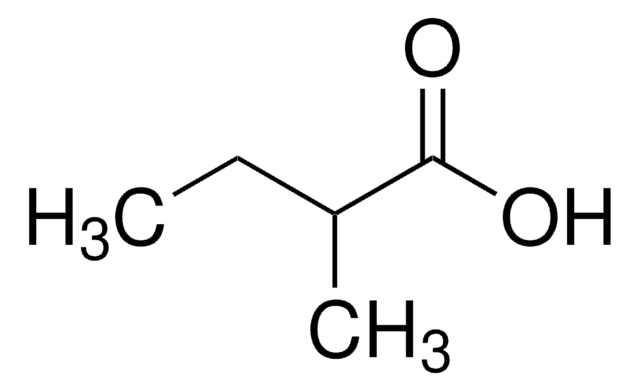
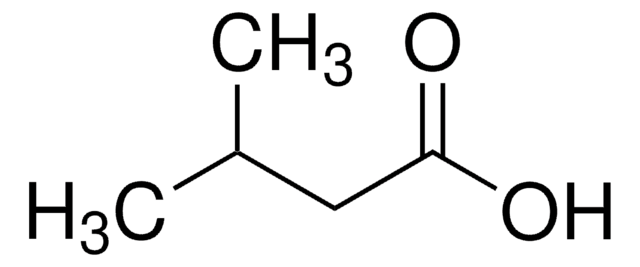
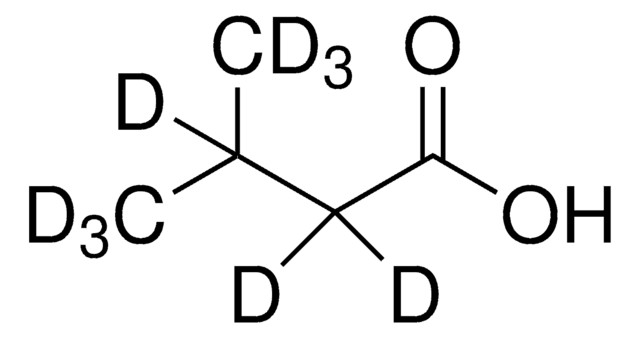
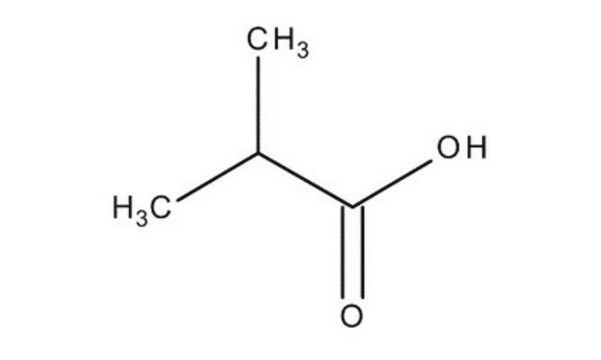
129542
Isovaleric acid
99%
Synonym(s):
3-Methylbutanoic acid, 3-Methylbutyric acid
About This Item
Recommended Products
vapor pressure
0.38 mmHg ( 20 °C)
Quality Level
Assay
99%
form
liquid
autoignition temp.
824 °F
refractive index
n20/D 1.403 (lit.)
bp
175-177 °C (lit.)
mp
−29 °C (lit.)
density
0.925 g/mL at 20 °C (lit.)
SMILES string
CC(C)CC(O)=O
InChI
1S/C5H10O2/c1-4(2)3-5(6)7/h4H,3H2,1-2H3,(H,6,7)
InChI key
GWYFCOCPABKNJV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
176.0 °F - Pensky-Martens closed cup
Flash Point(C)
80 °C - Pensky-Martens closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Inborn errors of metabolism are caused by changes in specific enzymatic reactions and hundreds of different such alterations, which affect about 1 of every 5000 newborns, have been characterized.
Separation of Propionic acid; Acetic acid; Heptanoic acid; Isobutyric acid; Valeric acid; Isocaproic acid; Butyric acid; Isovaleric acid
Separation of Methyl oleate; Caprylic acid; Heptanoic acid; Methyl decanoate; Methyl dodecanoate; Myristic acid; Methyl palmitate; Methyl palmitoleate; Methyl stearate; Methyl linoleate; Methyl linolenate; Acetic acid; Arachidic acid; Behenic acid; Propionic acid; Isobutyric acid; Valeric acid; Isovaleric acid; Isocaproic acid; Butyric acid
Today, diverse studies report the benefits of probiotics, such as inhibitory effects on pathogens, aid in the management or prevention of chronic intestinal inflammatory diseases or atopic syndromes, and support to the immune system. Potential beneficial applications abound, researchers continue to evaluate the effictiveness and clarify the mechanisms of action of probiotics.
Protocols
In this study, SPME was used for the analysis of free fatty acids in Parmesan cheese using a 65 μm Carbowax/divinylbenzene (DVB) SPME fiber. Headspace extraction of the cheese sample was conducted at 65 °C for 15 minutes and analyzed by GC with FID detection. SPME is ideal for analyzing the volatiles associated with solid food samples. The phase chemistry of the Nukol GC column provides excellent peak shape of acidic compounds.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service