All Photos(1)
About This Item
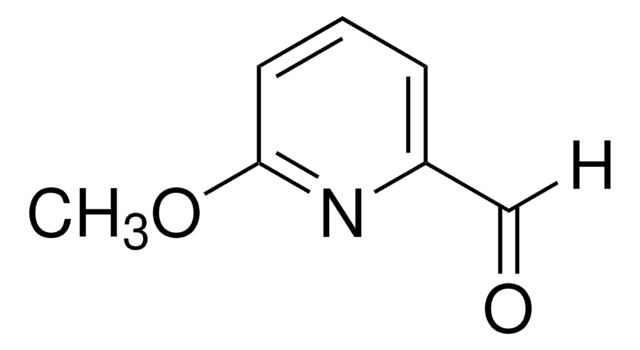
Empirical Formula (Hill Notation):
C7H6N2O2
CAS Number:
Molecular Weight:
150.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
mp
204-206 °C (lit.)
SMILES string
O=C1COc2cccnc2N1
InChI
1S/C7H6N2O2/c10-6-4-11-5-2-1-3-8-7(5)9-6/h1-3H,4H2,(H,8,9,10)
InChI key
ANHQLUBMNSSPBV-UHFFFAOYSA-N
Application
2H-Pyrido[3,2-b]-1,4-oxazin-3(4H)-one was used in the synthesis of quinazolinbenzoxazine derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Margarete von Wantoch Rekowski et al.
Bioorganic & medicinal chemistry, 18(3), 1288-1296 (2009-12-29)
Soluble guanylyl cyclase (sGC) is an ubiquitously expressed enzyme that generates the second messenger cGMP and hence, leads to a number of physiological responses including vasodilation, inhibition of platelet aggregation and neurotransmission. Whilst many activating and stimulating modulators of sGC
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![6-Iodo-3,4-dihydro-2H-pyrano[3,2-b]pyridine-8-carbaldehyde AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/372/131/71a9c521-75e7-431b-982b-1900f5334f95/640/71a9c521-75e7-431b-982b-1900f5334f95.png)
![2-Amino-1,3,8-triazaspiro[4.5]dec-1-en-4-one dihydrochloride AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/331/817/db18f9c8-f58a-4b59-86c1-cb26f4856702/640/db18f9c8-f58a-4b59-86c1-cb26f4856702.png)