125423
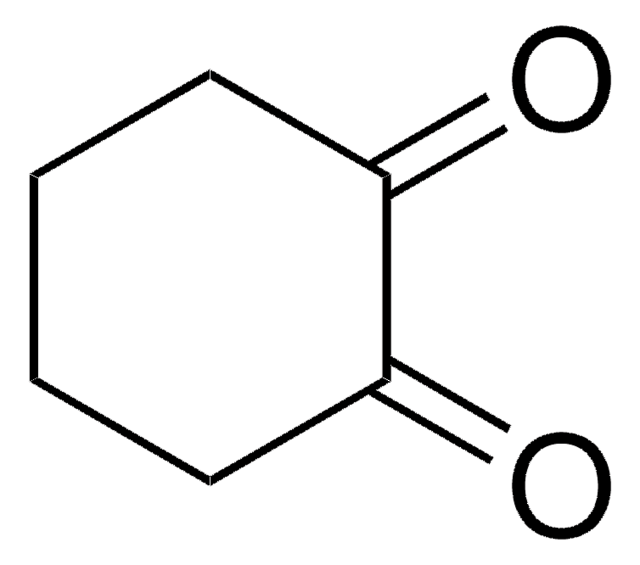
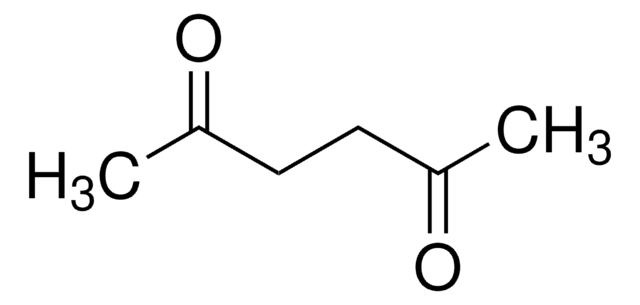
1,4-Cyclohexanedione
98%
Synonym(s):
1,4-Dioxocyclohexane, Cyclohexan-1,4-dione, Tetrahydroquinone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H8(=O)2
CAS Number:
Molecular Weight:
112.13
Beilstein:
774152
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
77-78.5 °C (lit.)
SMILES string
O=C1CCC(=O)CC1
InChI
1S/C6H8O2/c7-5-1-2-6(8)4-3-5/h1-4H2
InChI key
DCZFGQYXRKMVFG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,4-Cyclohexanedione(CHD) undergoes uncatalyzed oscillatory reactions during oxidation by acidic bromate in nitric acid and sulphuric acid solution. It reacts with acidic bromate to form 1,4-dihydroxybenzene which on further oxidation and bromination yields 1,4-benzoquinone and bromoorganics.
Application
1,4-Cyclohexanedione has been used to study the influence of visible light on the bromate-1,4-cyclohexanedione-ferroin oscillating reaction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
269.6 °F - closed cup
Flash Point(C)
132 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Uncatalysed oscillatory chemical reactions. Oxidation of 1, 4-cyclohexanedione by bromate in sulfuric or nitric acid solution.
Farage VJ and Janjic D.
Chemical Physics Letters, 88(3), 301-304 (1982)
Photosensitive, bubble-free, bromate-1, 4-cyclohexanedione oscillating reactions. Illumination control of pattern formation.
Kurin-Csorgei K, et al.
The Journal of Physical Chemistry A, 101(37), 6827-6829 (1997)
The 1, 4-cyclohexanedione-bromate-acid oscillatory system. 3. Detailed mechanism.
Szalai I and Koros E.
The Journal of Physical Chemistry A, 102(35), 6892-6897 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service