538205
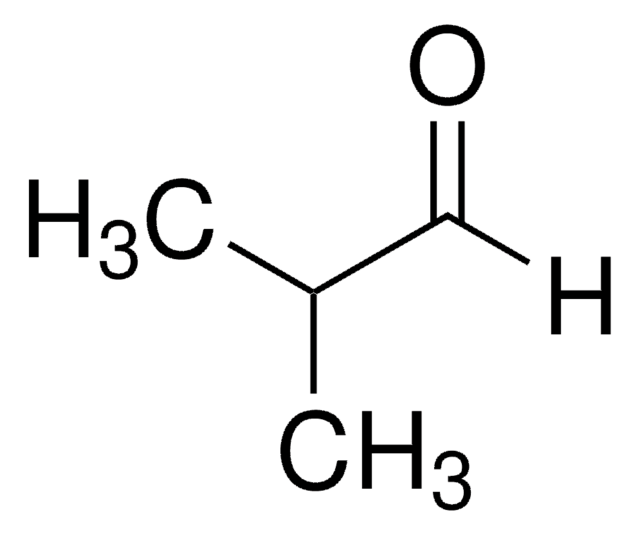
Isobutyraldehyde
dry, 98%
Synonym(s):
2-Methylpropanal, 2-Methylpropionaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2CHCHO
CAS Number:
Molecular Weight:
72.11
Beilstein:
605330
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
2.5 (vs air)
Quality Level
vapor pressure
66 mmHg ( 4.4 °C)
Assay
98%
autoignition temp.
384 °F
expl. lim.
10 %, 25 °F
2 %, 32 °F
refractive index
n20/D 1.374 (lit.)
bp
63 °C (lit.)
mp
−65 °C (lit.)
solubility
60 g/L at 25 °C
density
0.79 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]C(=O)C(C)C
InChI
1S/C4H8O/c1-4(2)3-5/h3-4H,1-2H3
InChI key
AMIMRNSIRUDHCM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Isobutyraldehyde is an α,α-disubstituted aldehyde. It is an important raw material for the manufacture of various industrially important chemicals. It may be produced from CO2 and overexpression of ribulose 1,5-bisphosphate carboxylase/oxygenase employing genetically engineered strain of Synechococcus elongatus PCC7942. Enantioenriched γ-nitroaldehydes are obtained from the conjugate addition reaction between isobutyraldehyde with nitroalkenes in the presence of chiral organocatalysts.
Application
Isobutyraldehyde may be used in the synthesis of the following compounds:
- N-cyclohrxyl-α-dimethylaminoisovalerarnide
- N′-cyclohexyl-N,N-dimethyl-α-dimethylaminoisovaleramidine
- α-acetoxy-N-cyclohexylisovaleramide
- acetic anhydride
- N-cyclohexyl-a-formyloxyisovaleramide
- cyclic carbonates
- hydroxypivaldehyde, which is useful for the preparation of neopentyl glycol (NPG)
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
-11.2 °F - closed cup
Flash Point(C)
-24 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reactions of cyclohexylisonitrile and isobutyraldehyde with various nucleophiles and catalysts.
McFarland JW.
The Journal of Organic Chemistry, 28(9), 2179-2181 (1963)
Cross-Aldol condensation of isobutyraldehyde and formaldehyde using phase transfer catalyst.
Hashmi A.
Journal of Saudi Chemical Society (2013)
Enantioselective Michael addition of isobutyraldehyde to nitroalkenes organocatalyzed by chiral primary amine-guanidines.
Avila A, et al.
Tetrahedron Asymmetry, 25(5), 462-467 (2014)
Xiang Liu et al.
Journal of biotechnology, 164(2), 188-195 (2012-09-15)
We have determined the X-ray crystal structures of the NADH-dependent alcohol dehydrogenase LlAdhA from Lactococcus lactis and its laboratory-evolved variant LlAdhA(RE1) at 1.9Å and 2.5Å resolution, respectively. LlAdhA(RE1), which contains three amino acid mutations (Y50F, I212T, and L264V), was engineered
Bettina M Pause et al.
Psychophysiology, 40(2), 209-225 (2003-06-25)
The aim of the present study was to investigate the similarities and differences in the olfactory and visual processing of emotional stimuli in healthy subjects and in patients with major depressive disorder (MDD). Twenty-five inpatients were investigated after admission to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service