115959
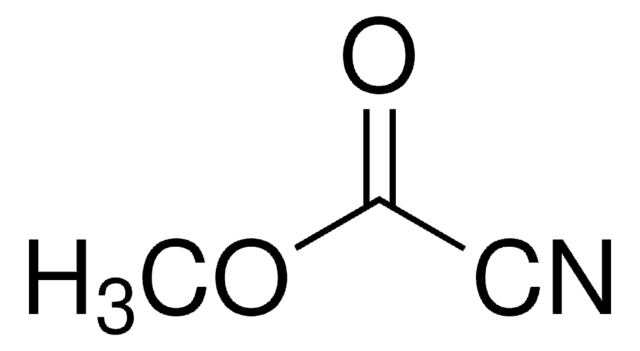
Benzoyl cyanide
98%
Synonym(s):
Phenylglyoxylonitrile
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5COCN
CAS Number:
Molecular Weight:
131.13
Beilstein:
1072101
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
206 °C (lit.)
mp
28-31 °C (lit.)
density
1.106 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
O=C(C#N)c1ccccc1
InChI
1S/C8H5NO/c9-6-8(10)7-4-2-1-3-5-7/h1-5H
InChI key
GJQBHOAJJGIPRH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Benzoyl cyanide is used to study the mechanism of reduction of benzoyl cyanide in acetonitrile, N,N-dimethylformamide and acetonitrile. It undergoes hydrolysis by Rhodococcus sp. CCZU10-1 to form benzoylformic acid.
Application
Reagent for selective acylation of amino compounds.
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
183.2 °F - closed cup
Flash Point(C)
84 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, 433-433 (1993)
Weisi Xiong et al.
Biotechnology letters, 37(8), 1703-1709 (2015-04-22)
To develop a biphasic system for use in plant tissue-mediated biotransformations to overcome the low water solubilities of substrates and inhibitory effects of products. Commonly-used organic solvents and ionic liquid were assayed using the biphasic system to reduce water-insoluble benzoyl
Yi Xu et al.
Journal of virology, 92(11) (2018-03-09)
Translational readthrough of the stop codon of the capsid protein (CP) open reading frame (ORF) is used by members of the Luteoviridae to produce their minor capsid protein as a readthrough protein (RTP). The elements regulating RTP expression are not
Petar I Penev et al.
Genome biology and evolution, 12(10), 1694-1710 (2020-08-14)
The ribosome's common core, comprised of ribosomal RNA (rRNA) and universal ribosomal proteins, connects all life back to a common ancestor and serves as a window to relationships among organisms. The rRNA of the common core is similar to rRNA
Norma A Macías-Ruvalcaba et al.
The Journal of organic chemistry, 72(2), 589-594 (2007-01-16)
The mechanism of reduction of benzoyl cyanide, 6, p-methoxybenzoyl cyanide, 7, and p-chlorobenzoyl cyanide, 8, has been studied in acetonitrile (6 and 7), N,N-dimethylformamide (6), and acetonitrile containing water (all three compounds). The reaction proceeds by initial reduction to form
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service