All Photos(1)
About This Item
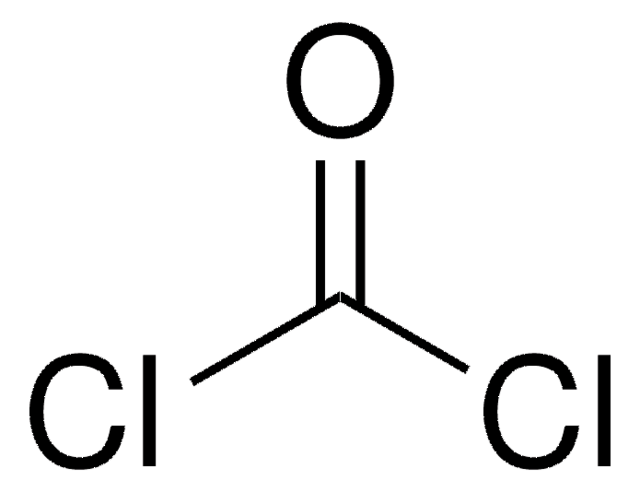
Linear Formula:
CSCl2
CAS Number:
Molecular Weight:
114.98
Beilstein:
1633495
EC Number:
MDL number:
UNSPSC Code:
12352000
eCl@ss:
38020402
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
4 (vs air)
Quality Level
reaction suitability
reaction type: Carbonylations
refractive index
n20/D 1.548 (lit.)
bp
70-75 °C (lit.)
density
1.50 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
ClC(Cl)=S
InChI
1S/CCl2S/c2-1(3)4
InChI key
ZWZVWGITAAIFPS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
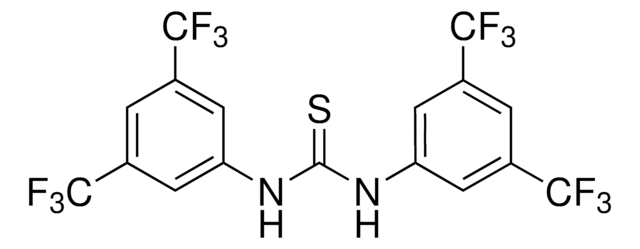
Reagent used to prepare a bulky thiourea ligand for palladium-catalyzed aerobic oxidation of alcohols to aldehydes and ketones.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
W Kedzierski et al.
The Journal of chemical physics, 120(19), 9087-9089 (2004-07-23)
A special xenon matrix detector has been used to study the production of S(1S) following controlled electron impact on thiophosgene (Cl2CS) targets over an electron energy range from threshold to 400 eV. Time-of-flight spectroscopy has been used to measure S(1S)
Christof Jung et al.
The journal of physical chemistry. A, 110(16), 5317-5325 (2006-04-21)
The dispersed fluorescence spectrum of the ground electronic state of thiophosgene, SCCl2, is analyzed in a very complex region of vibrational excitation, 7000-9000 cm(-1). The final result is that most of the inferred excited vibrational levels are assigned in terms
Cheng Li et al.
Colloids and surfaces. B, Biointerfaces, 171, 159-166 (2018-07-22)
Dual mode imaging technology is widely developed to achieve the early-stage precision cancer diagnosis. Here we designed a dual-modal magnetic resonance/near infrared fluorescence optical imaging contrast agent (GdF-SS-NIR783) with the fluorescence activatable and safer gadofullerene. The nanoprobes were fabricated by
Youlia Hagooly et al.
The Journal of organic chemistry, 73(17), 6780-6783 (2008-08-13)
A general preparation for aromatic and aliphatic, cyclic as well as linear, symmetric and asymmetric difluoromethylenedioxy derivatives is described. The alcohols were reacted with thiophosgene to give thiocarbonates, which in turn were reacted with BrF3. The fluorination step is complete
T A Lewis et al.
Journal of bacteriology, 177(8), 2204-2208 (1995-04-01)
Pseudomonas sp. strain KC transforms carbon tetrachloride into carbon dioxide and nonvolatile products, without chloroform as an intermediate. To define the pathway for hydrolysis, nonvolatile products were analyzed. Condensation products containing the carbon atom of carbon tetrachloride as carbonyl and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service