U4885
UNC0638 hydrate
≥98% (HPLC)
Synonym(s):
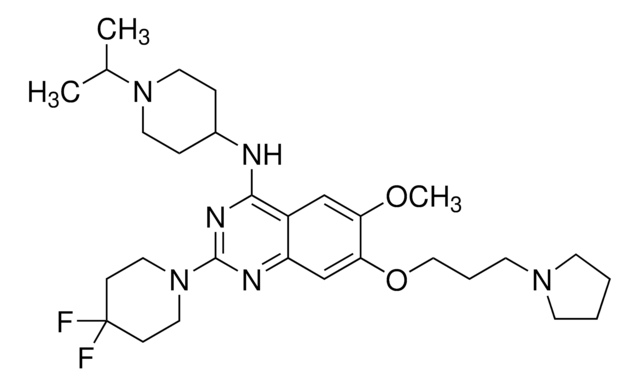
2-Cyclohexyl-N-(1-isopropylpiperidin-4-yl)-6-methoxy-7-(3-(pyrrolidin-1-yl)propoxy) quinazolin-4-amine
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
solid
storage condition
protect from light
color
off-white
solubility
DMSO: >10 mg/mL
storage temp.
−20°C
SMILES string
O.COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN4CCCC4)C5CCCCC5
InChI
1S/C30H47N5O2.H2O/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23;/h20-24H,4-19H2,1-3H3,(H,31,32,33);1H2
InChI key
LLJGACAJGYXBTL-UHFFFAOYSA-N
General description
Application
- in fractionation and isolation of human blood cells and cell nuclei
- in the analysis of human immunodeficiency virus (HIV) reactivation in latently infected cell line
- to determine the effect of G9a and its enzymatic activity on cisplatin resistance
Biochem/physiol Actions
To learn about other SGC chemical probes for epigenetic targets, visit sigma.com/sgc
Features and Benefits
Other Notes
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Aquatic Chronic 4
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Epigenetic modifications are thought to occur through two key interconnected processes—DNA methylation and the covalent modification of histones.
Related Content
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service