T8658
Trypsin from bovine pancreas
suitable for protein sequencing, lyophilized powder
Synonym(s):
Porcine Trypsin, Trypsin for Mass Spectropetry
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
grade
Proteomics Grade
Quality Level
form
lyophilized powder
mol wt
23.8 kDa
packaging
vial of 100 μg
solubility
hydrochloric acid: soluble 1 mM, clear
suitability
suitable for protein sequencing
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Trypsin from bovine pancreas has been used for in-gel digestion for MS (mass spectrometry) analysis.
- It has been used for the digestion of albumin for size-exclusion chromatography.
- It has been used for the digestion of HDL (high density lipoprotein) for LC-MS (liquid chromatography-mass spectrometry) analysis.
- It has been used for limited proteolysis of IST1 (putative MAPK-activating protein PM28).
For trypsin digestion of peptides, use a ratio of about 1:100 to 1:20 for trypsin:peptide. The typical use for this product is in removing adherent cells from a culture surface. The concentration of trypsin necessary to dislodge cells from their substrate is dependent primarily on the cell type and the age of the culture. Trypsins have also been used for the re-suspension of cells during cell culture, in proteomics research for digestion of proteins and in various in-gel digestions. Additional applications include assessing crystallization by membrane-based techniques and in a study to determine that protein folding rates and yields can be limited by the presence of kinetic traps.
Biochem/physiol Actions
Trypsin cleaves peptides on the C-terminal side of lysine and arginine residues. The rate of hydrolysis of this reaction is slowed if an acidic residue is on either side of the cleavage site and hydrolysis is stopped if a proline residue is on the carboxyl side of the cleavage site. The optimal pH for trypsin activity is 7-9. Trypsin can also act to cleave ester and amide linkages of synthetic derivatives of amino acids. EDTA is added to trypsin solutions as a chelating agent that neutralizes calcium and magnesium ions that obscure the peptide bonds on which trypsin acts. Removing these ions increases the enzymatic activity.
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
Components
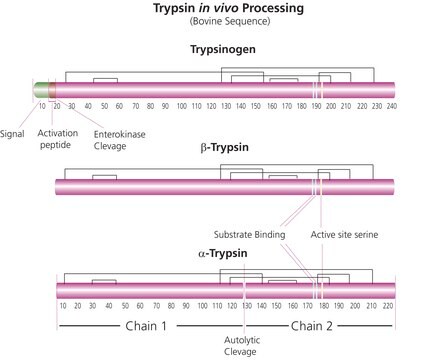
Trypsin consists of a single chain polypeptide of 223 amino acid residues, produced by the removal of the N-terminal hexapeptide from trypsinogen which is cleaved at the Lys - lle peptide bond. The sequence of amino acids is cross-linked by 6 disulfide bridges. This is the native form of trypsin, beta-trypsin. BETA-trypsin can be autolyzed, cleaving at the Lys - Ser residue, to produce alpha-trypsin. Trypsin is a member of the serine protease family.
Caution
Solutions in 1 mM HCl are stable for 1 year in aliquots and stored at -20°C. The presence of Ca2+ will also diminish the self-autolysis of trypsin and maintain its stability in solution. Trypsin will also retain most of its activity in 2.0 M urea, 2.0 M guanidine HCl, or 0.1% (w/v) SDS.
Unit Definition
One BAEE unit will produce a A253 of 0.001 per minute at pH 7.6 at 25°C using BAEE as a substrate.
Preparation Note
This product is from pancreas sourced from New Zealand. It is soluble in 1 mM HCl at 1 mg/mL.
inhibitor
Product No.
Description
Pricing
substrate
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D M Eckert et al.
Cell, 99(1), 103-115 (1999-10-16)
The HIV-1 gp41 protein promotes viral entry by mediating the fusion of viral and cellular membranes. A prominent pocket on the surface of a central trimeric coiled coil within gp41 was previously identified as a potential target for drugs that
Anthony G W Norden et al.
Kidney international, 66(5), 1994-2003 (2004-10-22)
Quantitative data on protein and polypeptide excretion in normal urine are lacking. In Fanconi syndrome, failure of proximal tubular protein reabsorption leads to 'tubular' proteinuria, but little is known about peptide excretion. Urine from normal (N=5) and Fanconi patients (Dent's
Conformational Changes in the Endosomal Sorting Complex Required for the Transport III Subunit Ist1 Lead to Distinct Modes of ATPase Vps4 Regulation.
Tan J, et al.
The Journal of Biological Chemistry, 290, 30053-30053 (2015)
Shanjin Huang et al.
The Journal of biological chemistry, 279(22), 23364-23375 (2004-03-25)
The cytoskeleton is a key regulator of plant morphogenesis, sexual reproduction, and cellular responses to extracellular stimuli. During the self-incompatibility response of Papaver rhoeas L. (field poppy) pollen, the actin filament network is rapidly depolymerized by a flood of cytosolic
J S Gilchrist et al.
The Journal of biological chemistry, 268(6), 4291-4299 (1993-02-25)
Recent evidence suggests that nuclei possess Ca2+ transport mechanisms to regulate nucleoplasmic/cytosolic Ca2+ gradients. We, therefore, investigated the possibility that Ca(2+)-binding proteins may also exist within the nucleus. Electrophoretic analysis revealed the presence of an acidic 93-kDa protein (p93) in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service