SRP3271
GM-CSF rat
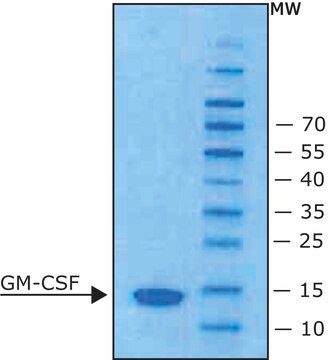
Animal-component free, recombinant, expressed in E. coli, ≥98% (SDS-PAGE), ≥98% (HPLC)
Synonym(s):
CSF-3, GM-CSF?, MGI-1G, pluripoietin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352202
NACRES:
NA.75
Recommended Products
biological source
rat
recombinant
expressed in E. coli
Assay
≥98% (HPLC)
≥98% (SDS-PAGE)
form
lyophilized
potency
≥0.01 ng/mL
mol wt
14.5 kDa
packaging
pkg of 20 μg
impurities
<0.1 EU/μg endotoxin, tested
color
white to off-white
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
rat ... CSF2(12981)
General description
It is produced in by endothelial cells, monocytes, fibroblasts and T-lymphocytes. GM-CSF inhibits neutrophil migration and enhances the functional activity of the mature end-cells. The human and murine molecules are species-specific and exhibit no cross-species reactivity.
Biochem/physiol Actions
GM-CSF is a hematopoietic growth factor that stimulates the development of neutrophils and macrophages and promotes the proliferation and development of early erythroid megakaryocytic and eosinophilic progenitor cells. Recombinant murine GM-CSF is a 14.5 kDa globular protein consisting of 128 amino acids residues.
Physical form
Lyophilized from 10 mM Tris, pH 8.5
Reconstitution
Centrifuge the vial prior to opening. Reconstitute in water to a concentration of 0.1-1.0 mg/mL. Do not vortex. This solution can be stored at 2-8°C for up to 1 week. For extended storage, it is recommended to further dilute in a buffer containing a carrier protein (example 0.1% BSA) and store in working aliquots at -20°C to -80°C.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J Nemunaitis et al.
Blood, 77(9), 2065-2071 (1991-05-01)
Forty-seven patients with hematologic neoplasia received recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) by daily 2-hour infusion following allogeneic bone marrow transplantation from HLA-identical sibling donors in a phase I-II dose-escalation trial. Dose levels ranged from 30 to 500 micrograms/m2/d. At
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service