SML3568
C26-A6
≥98% (HPLC)
Synonym(s):
5-chloro-2-methoxy-N-(2-methyl-[1,2,4]triazolo[1,5-a]pyridin-8-yl)benzenesulfonamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H13ClN4O3S
CAS Number:
Molecular Weight:
352.80
UNSPSC Code:
12352200
NACRES:
NA.21
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 2 mg/mL, clear (Warmed)
storage temp.
2-8°C
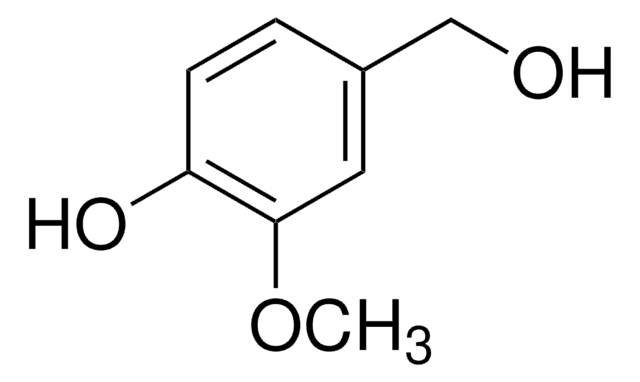
SMILES string
O=S(NC1=CC=CN2N=C(C)N=C12)(C3=CC(Cl)=CC=C3OC)=O
Biochem/physiol Actions
A26-A6 is a MTDH-SND1 interaction blocker (IC50 = 2.4 μM by cell-free & 12.3 μM by cellular split-luc assay) that competes against metadherin (MTDH; AEG-1; LYRIC) for staphylococcal nuclease domain-containing 1 (SND1) binding, thereby preventing MTDH-SND1 complex from suppressing antitumor T cell responses. A26-A6 inhibits PyMT tumor sphere formation in cultures (IC50 <200 μM) in a MTDH- and SND1-dependent manner and exhibits in vivo efficacy against breast tumor growth and metastasis in mice in vivo (15 mg/kg/d i.v. 5d/wk), including the murine PyMT model, and human breast cancer xenografts (PDX, HCI-001, SCP28).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Minhong Shen et al.
Nature cancer, 3(1), 60-74 (2022-02-06)
Despite increased overall survival rates, curative options for metastatic breast cancer remain limited. We have previously shown that metadherin (MTDH) is frequently overexpressed in poor prognosis breast cancer, where it promotes metastasis and therapy resistance through its interaction with staphylococcal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service