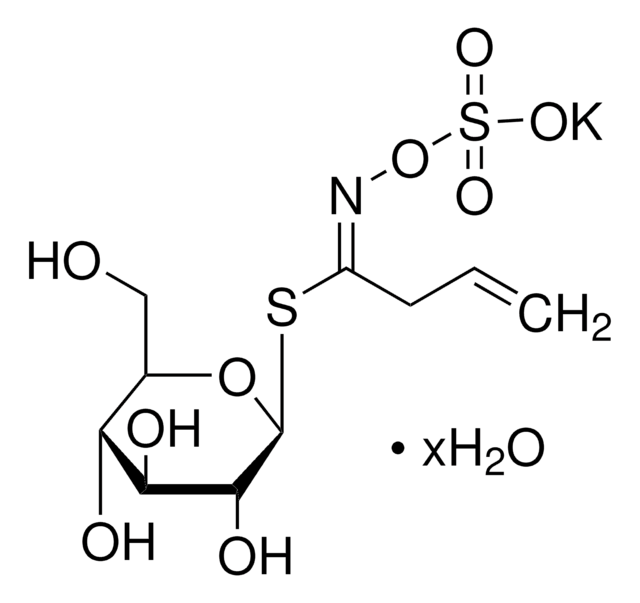
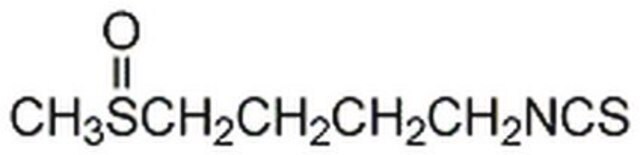S4441
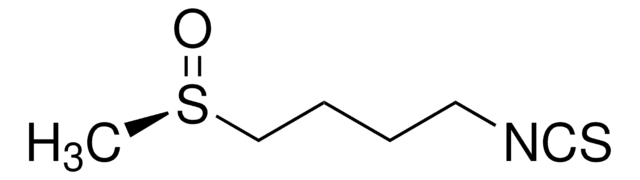
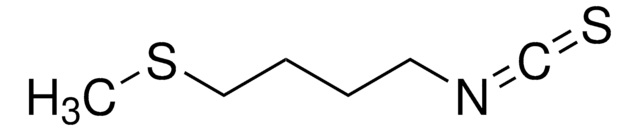
D,L-Sulforaphane
≥90% (HPLC), liquid, phase II detoxification enzymes inducer
Synonym(s):
1-Isothiocyanato-4-(methylsulfinyl)-butane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11NOS2
CAS Number:
Molecular Weight:
177.29
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Product Name
DL-Sulforaphane, ≥90% (HPLC), synthetic, liquid
biological source
synthetic
Assay
≥90% (HPLC)
form
liquid
solubility
DMSO: soluble 40 mg/mL
H2O: insoluble
compatibility
for use with ABI 7500
shipped in
dry ice
storage temp.
−20°C
SMILES string
CS(=O)CCCCN=C=S
InChI
1S/C6H11NOS2/c1-10(8)5-3-2-4-7-6-9/h2-5H2,1H3
InChI key
SUVMJBTUFCVSAD-UHFFFAOYSA-N
Application
LNCaP prostate cancer cells were treated with DL-Sulforaphane to study the effect on androgen receptor. Effects on malathion toxicity was studied in rats by treating them with DL-Sulforaphane.
Biochem/physiol Actions
Selective inducer of phase II detoxification enzymes with anticarcinogenic properties. Organosulfur compound found in cruciferous vegetables, including broccoli.
Sulforaphane is an anti-cancer, anti-microbial and anti-diabetic compound found in cruciferous vegetables. It induces the production of detoxifying enzymes such as quinone reductase and glutathione S-transferase that cause xenobiotic transformation. Sulforaphane also increases the transcription of tumor suppressor proteins and inhibits histone deacetylases. It modulates inflammatory responses by suppressing the LPS-mediated expression of iNOS, COX-2, IL-1β and TNF-α.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Kenneth MacLeod et al.
British journal of cancer, 115(12), 1530-1539 (2016-11-09)
Although the nuclear factor-erythroid 2-related factor 2 (NRF2) pathway is one of the most frequently dysregulated in cancer, it is not clear whether mutational status is a good predictor of NRF2 activity. Here we utilise four members of the aldo-keto
Verena Jeschke et al.
Frontiers in plant science, 8, 1995-1995 (2017-12-07)
Multiple lepidopteran larvae feed successfully on plants containing glucosinolates despite the diverse array of toxic and deterrent breakdown products, such as isothiocyanates (ITCs), formed upon plant damage. While much is known about how specialist lepidopterans metabolize and tolerate glucosinolates, there
Carmela Fimognari et al.
Mutation research, 635(2-3), 90-104 (2006-12-01)
A number of natural compounds with inhibitory effects on tumorigenesis have been identified from our diet. Several studies have documented the cancer-preventive activity of a significant number of isothiocyanates (ITCs), the majority of which occur in plants, especially in Cruciferous
Masayoshi Yamaguchi et al.
International journal of molecular medicine, 38(6), 1940-1946 (2016-11-15)
β-caryophyllene, which is a constituent of many essential oils, has been known to be a selective agonist of the cannabinoid receptor type-2 and to exert cannabimimetic anti-inflammatory effects in animals. The effects of β-caryophyllene on macrophages have not been investigated
Systems Approach Reveals Nuclear Factor Erythroid 2-Related Factor 2/Protein Kinase R Crosstalk in Human Cutaneous Leishmaniasis.
Áislan de Carvalho Vivarini et al.
Frontiers in immunology, 8, 1127-1127 (2017-09-30)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service