30960
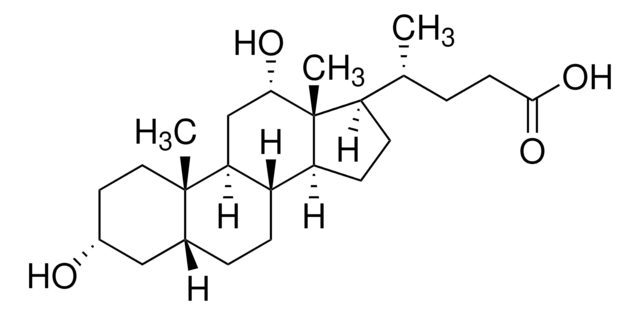
Deoxycholic acid
≥99.0% (T)
Synonym(s):
3α,12α-Dihydroxy-5β-cholanic acid, 7-Deoxycholic acid, Desoxycholic acid
About This Item
Recommended Products
biological source
ox bile
Quality Level
description
anionic
Assay
≥99.0% (T)
form
powder
optical activity
[α]20/D +54±1°, c = 1% in ethanol
mol wt
392.57 g/mol
impurities
≤1% cholic acid (TLC)
mp
170-175 °C
171-174 °C (lit.)
functional group
carboxylic acid
SMILES string
C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C
InChI
1S/C24H40O4/c1-14(4-9-22(27)28)18-7-8-19-17-6-5-15-12-16(25)10-11-23(15,2)20(17)13-21(26)24(18,19)3/h14-21,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15-,16-,17+,18-,19+,20+,21+,23+,24-/m1/s1
InChI key
KXGVEGMKQFWNSR-LLQZFEROSA-N
Gene Information
human ... ATIC(471)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- a hyphal-inhibitory compound in an ex vivo assay to study the hyphal morphogenesis of Candida albicans in the gastrointestinal tract
- an internal standard to estimate the toxin concentration in the cell pellet
- a bile acid (BA) standard to quantitatively measure BA in various samples from treated mice by ultra-performance liquid chromatography triple quadrupole mass spectrometry
Biochem/physiol Actions
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service