H2766
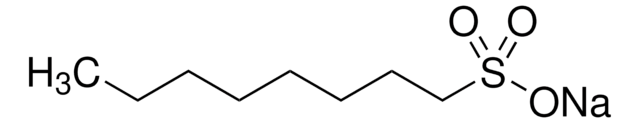
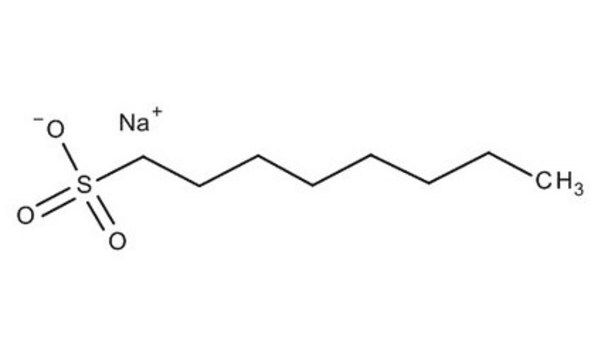
Sodium 1-heptanesulfonate
Synonym(s):
1-Heptanesulfonic acid sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C7H15O3SNa
CAS Number:
Molecular Weight:
202.25
EC Number:
MDL number:
UNSPSC Code:
12161900
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
biological source
synthetic (oragnic)
Quality Level
description
anionic
form
powder
mol wt
202.25 g/mol
technique(s)
HPLC: suitable
LC/MS: suitable
impurities
<2.0% water (Karl Fischer)
mp
300 °C (572 °F)
solubility
H2O: ≥100 mg/mL
cation traces
Na: 10.8-12.0 % (w/v) (anhydrous)
SMILES string
[Na+].CCCCCCCS([O-])(=O)=O
InChI
1S/C7H16O3S.Na/c1-2-3-4-5-6-7-11(8,9)10;/h2-7H2,1H3,(H,8,9,10);/q;+1/p-1
InChI key
REFMEZARFCPESH-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Sodium 1-heptanesulfonate belongs to the sodium alkylsulfonate group of surfactants. It has a -SO?3 head group with sodium and an alkyl chain attached to the head group. Sodium 1-heptanesulfonate has a critical micelle concentration value of 0.302 mol/dm3.
Sodium 1-heptanesulfonate, also known as sodium heptanesulfonate, is a versatile compound commonly used in lab experiments and biochemical research. It acts as an ion-pairing agent, surfactant, and buffer, playing a crucial role in various applications. In biochemistry, it helps with tasks like protein purification and nucleic acid separation by reducing surface tension for better mixing. It also serves as a chelating agent for metal ion analysis and acts as a buffer in biological experiments. Sodium 1-heptanesulfonate is a preferred choice in HPLC and high-performance capillary electrophoresis analyses, aiding in the separation of positively charged analytes in both organic and inorganic compounds, from pharmaceuticals to peptide research.
Application
Sodium 1-heptanesulfonate has been used as a component of the mobile phase buffer for preequilibration of high performance liquid chromatography column.
Ion-pairing reagent for HPLC, including analyses of peptides and proteins.
Biochem/physiol Actions
Sodium 1-heptanesulfonate serves as an ion-pairing reagent for analysis of epinephrine and norepinephrine in ion-pairing chromatography (IPC).
Features and Benefits
- Ideal for Cell Biology and Biochemical Research
- High purity chemical suitable for multiple research applications
Other Notes
For additional information on our range of Biochemicals, please complete this form.
comparable product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Validated HPLC-Fl method for the analysis of S-adenosylmethionine and S-adenosylhomocysteine biomarkers in human blood
Albu C, et al.
Journal of Fluorescence, 23(3), 381-386 (2013)
Determination of S-adenosylmethionine and S-adenosylhomocysteine from human blood samples by HPLC-FL
Birsan C, et al.
Analytical Letters, 41(10), 1720-1731 (2008)
Analysis of catecholamines in urine by unique LC/MS suitable ion-pairing chromatography
Bergmann ML, et al.
Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 1057(2), 118-123 (2017)
A R Calhoun et al.
Journal of colloid and interface science, 309(2), 505-510 (2007-03-03)
Measurements have been made to determine the solubility of ethane, C2H6, in aqueous solutions of four different surfactants of the linear alkanesulfonate class at 25 degrees C. The surfactants, sodium 1-pentanesulfonate, sodium 1-hexanesulfonate, sodium 1-heptanesulfonate, and sodium 1-octanesulfonate, all share
Takeshi Oshizaka et al.
International journal of pharmaceutics, 475(1-2), 292-297 (2014-08-27)
Skin concentrations of topically administered compounds need to be considered in order to evaluate their efficacies and toxicities. This study investigated the relationship between the skin permeation and concentrations of compounds, and also predicted the skin concentrations of these compounds
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service