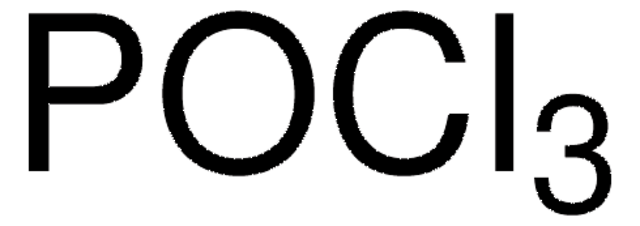About This Item
Recommended Products
vapor pressure
97 mmHg ( 20 °C)
Quality Level
product line
ReagentPlus®
Assay
≥99%
form
liquid
refractive index
n20/D 1.518 (lit.)
bp
79 °C (lit.)
mp
−105 °C (lit.)
density
1.631 g/mL at 25 °C (lit.)
anion traces
chloride (Cl-): 59.0-60.2%
SMILES string
ClS(Cl)=O
InChI
1S/Cl2OS/c1-4(2)3
InChI key
FYSNRJHAOHDILO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- To facilitate the synthesis of indenones from 3-hydroxyindanones via dehydroxylation.
- Surface modification of amorphous carbon nanotubes (α-CNTs) in stearic acid-dichloromethane solution.
- To functionalize silica gel for thioacetalization of aldehydes.
- Conversion of tert-butyl esters to acid chlorides.
- Conversion of aliphatic and aromatic sulfoxides to sulfides in the presence of triphenylphosphine.
- As an activator to generate ketenes in-situ for preparing 2-azetidinones.
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1A - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service