W271705
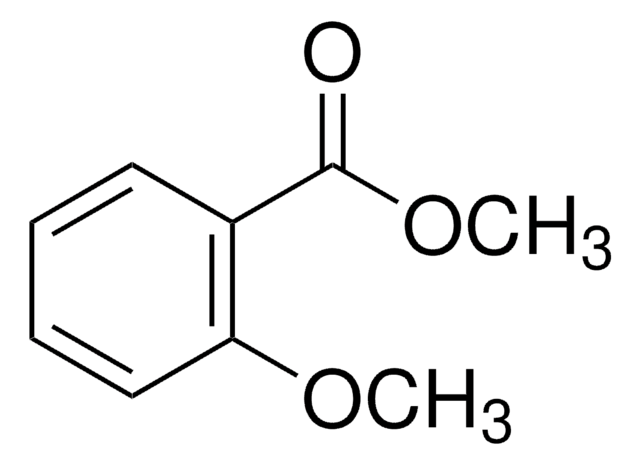
Methyl 2-methoxybenzoate
≥97%
Synonym(s):
Methyl o-anisate
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
Kosher
Agency
meets purity specifications of JECFA
Assay
≥97%
refractive index
n20/D 1.534 (lit.)
bp
248 °C (lit.)
density
1.157 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
hyacinth; herbaceous
SMILES string
COC(=O)c1ccccc1OC
InChI
1S/C9H10O3/c1-11-8-6-4-3-5-7(8)9(10)12-2/h3-6H,1-2H3
InChI key
PFYHAAAQPNMZHO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Characterizing Repellencies of Methyl Benzoate and Its Analogs against the Common Bed Bug, Cimex lectularius.: This study evaluates the repellent effects of Methyl 2-methoxybenzoate among other methyl benzoate analogs against bed bugs, highlighting its potential in developing non-toxic pest control solutions (Strickland et al., 2022).
- Copper-catalysed intramolecular O-arylation: a simple and efficient method for benzoxazole synthesis.: This research uses Methyl 2-methoxybenzoate as a ligand in the synthesis of benzoxazole, a compound with significant pharmaceutical applications, via copper-catalyzed reactions (Wu et al., 2014).
- Attraction of the orange mint moth and false celery leaftier moth (Lepidoptera: Crambidae) to floral chemical lures.: Explores the use of Methyl 2-methoxybenzoate in agricultural pest management, specifically its role in attracting and managing pest populations in crop fields (Landolt et al., 2014).
- Synthesis of (+/-)- and (-)-vibralactone and vibralactone C.: Discusses the synthetic applications of Methyl 2-methoxybenzoate in creating complex organic molecules, such as vibralactone, which are important in medicinal chemistry and biological research (Zhou and Snider, 2008).
Disclaimer
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service