P7251
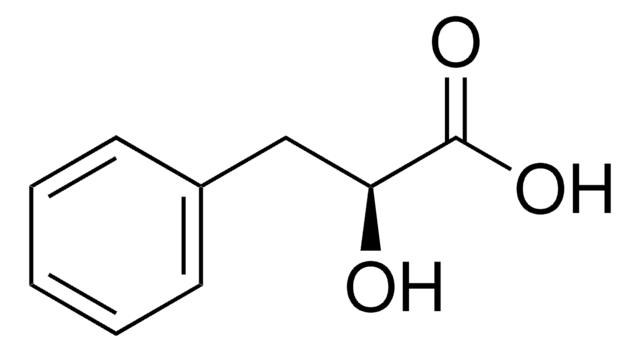
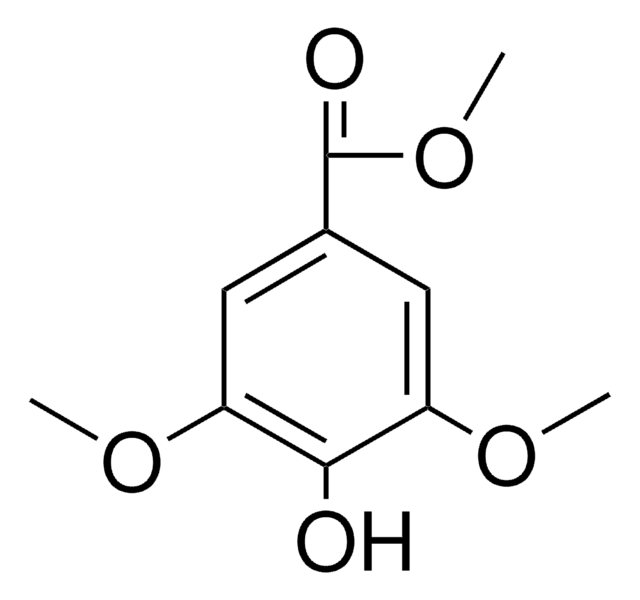
3-Phenyllactic acid
≥98%
Synonym(s):
α-Hydroxyhydrocinnamic acid, β-Phenyllactic acid, 2-Hydroxy-3-phenylpropionic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2CH(OH)COOH
CAS Number:
Molecular Weight:
166.17
Beilstein:
2209791
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
powder
SMILES string
OC(Cc1ccccc1)C(O)=O
InChI
1S/C9H10O3/c10-8(9(11)12)6-7-4-2-1-3-5-7/h1-5,8,10H,6H2,(H,11,12)
InChI key
VOXXWSYKYCBWHO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Phenyllactic acid can be used as a reactant to prepare:
- O
- -Acetyl-3-phenyllactic acid by reacting with acetic anhydride in the presence of pyridine.
- Ethyl 2-oxo-1-(phenylmethyl)-2-[(phenylmethyl)amino]ethyl carbonate by one pot reaction with 2-ethoxy-1-(ethoxycarbonyl)-1,2-dihydroquinoline (EEDQ) and aniline.
- Phenylmethyl-1,3-dioxolane-2,4-dione by condensation reaction with trichloromethyl chloroformate (diphosgene) in the presence of activated charcoal.
Reagent involved in biological studies of:
- Enantioselectivity of lipase in transesterification
- Oxidation by glycolate oxidase and catalase
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C N Sarkissian et al.
Analytical biochemistry, 280(2), 242-249 (2000-05-03)
Phenylketonuria (PKU) (OMIM 261600) is the first Mendelian disease to have an identified chemical cause of impaired cognitive development. The disease is accompanied by hyperphenylalaninemia (HPA) and elevated levels of phenylalanine metabolites (phenylacetate (PAA), phenyllactate (PLA), and phenylpyruvate (PPA)) in
Mattia Quattrini et al.
International journal of food microbiology, 302, 8-14 (2018-09-18)
Fungal spoilage of bread remains an unsolved issue in bread making. This work aims to identify alternative strategies to conventional preservatives in order to prevent or delay fungal spoilage of bread. The minimum inhibitory concentration (MIC) of bacterial metabolites and
S Iijima et al.
The Tohoku journal of experimental medicine, 117(2), 167-178 (1975-10-01)
Wister albino pregnant rats were fed on pellets containing 3.5% L-phenylalanine (Phe) from 10 days before the expected date of birth. The diet was then switched to 7% Phe pellets at the third week after birth. Baby rats were reared
Jessie Bong et al.
Food chemistry, 267, 355-367 (2018-06-24)
New Zealand manuka (Leptospermum scoparium) and kanuka (Kunzea ericoides) honeys contain a unique array of chemical markers useful for chemical fingerprinting. We investigated the presence of 13 potential marker compounds in nectars of the major honey crop species. We confirmed
Helena Lind et al.
FEMS microbiology letters, 271(2), 310-315 (2007-04-26)
Antifungal compounds from cultures of five type strains of dairy propionibacteria, as well as from the cultivation medium, were studied. Cell-free supernatants and medium were fractionated by C(18) solid phase extraction. The aqueous 95% acetonitrile fractions were analyzed by GC-MS
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service