M46209
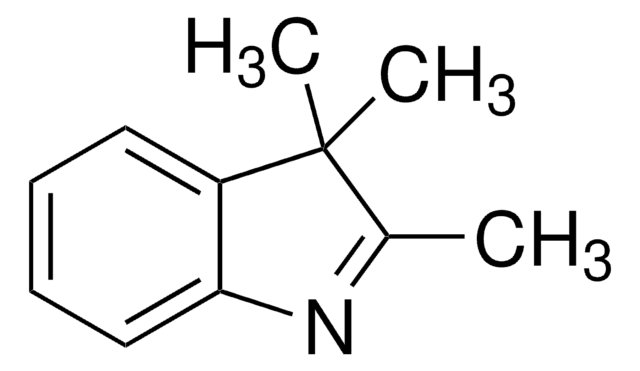
1,3,3-Trimethyl-2-methyleneindoline
97%
Synonym(s):
2-Methylene-1,3,3-trimethylindoline, Fischer base, NSC 66176
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H15N
CAS Number:
Molecular Weight:
173.25
Beilstein:
131162
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
liquid
Assay:
97%
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.577 (lit.)
bp
248 °C (lit.)
density
0.979 g/mL at 25 °C (lit.)
SMILES string
CN1C(=C)C(C)(C)c2ccccc12
InChI
1S/C12H15N/c1-9-12(2,3)10-7-5-6-8-11(10)13(9)4/h5-8H,1H2,2-4H3
InChI key
ZTUKGBOUHWYFGC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for:
- Cycloaddition reactions
- Preparation of red photochromic dyes
- Synthesis of photochromic homopolymers via ring-opening metathesis polymerization
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
145.4 °F - closed cup
Flash Point(C)
63 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Keum, S., et al.
Dyes and Pigments, 86, 74-74 (2010)
Ishmetova, R., I.; et al.
Heterocycles, 83, 1363-1363 (2011)
York, M.; Evans, R., A.
Tetrahedron Letters, 51, 2195-2195 (2010)
Maryam Raeesi et al.
Journal of colloid and interface science, 578, 379-389 (2020-06-15)
One of the biggest challenges in the field of photoresponsive spirooxazines is their fast reverse isomerization. Polar phase change materials beside spirooxazines not only stabilize their colored-form, but also induce thermo-regulating properties to the whole system. Moreover, encapsulation is a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1′,3′-Dihydro-1′,3′,3′-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2′-(2H)-indole] 98%](/deepweb/assets/sigmaaldrich/product/structures/503/745/147ecd2c-44b9-46e9-a8c9-bff9a2577218/640/147ecd2c-44b9-46e9-a8c9-bff9a2577218.png)

![1,1,2-Trimethylbenz[e]indole ≥98.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/296/735/4c0b92e3-1a5f-4c32-8b5b-0b0997c15df4/640/4c0b92e3-1a5f-4c32-8b5b-0b0997c15df4.png)

![1,3-Dihydro-1,3,3-trimethylspiro[2H-indole-2,3′-[3H]naphth[2,1-b][1,4]oxazine] ≥98.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/328/450/b7868d16-ffb3-47c0-8c9b-81a9630a963a/640/b7868d16-ffb3-47c0-8c9b-81a9630a963a.png)