F20505
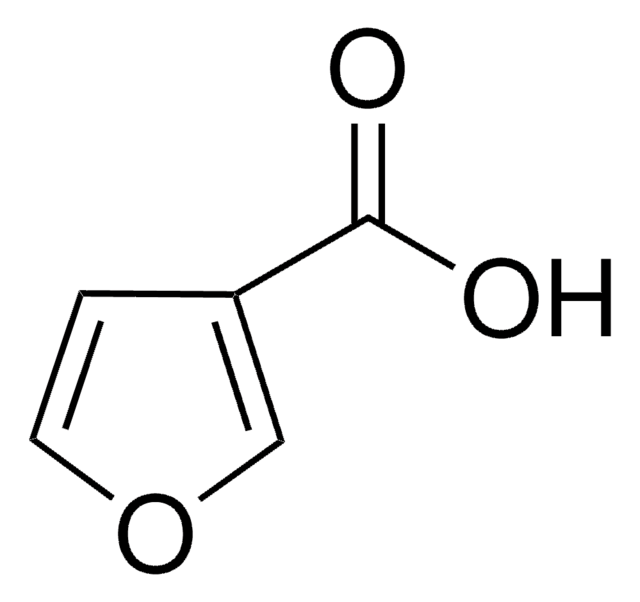
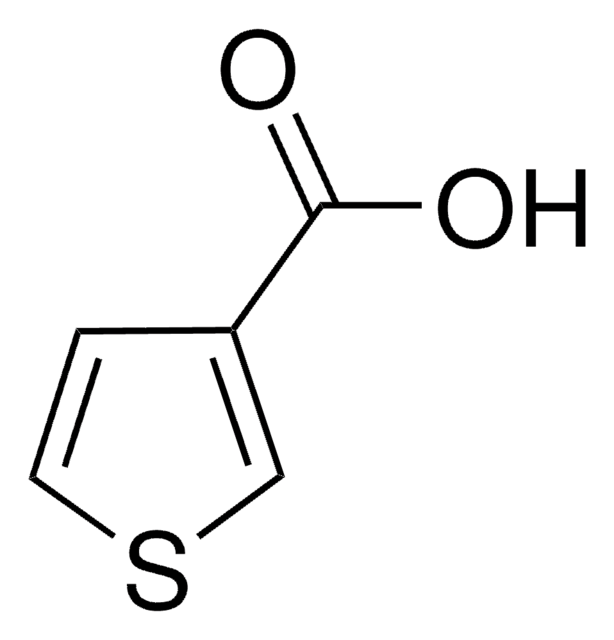
2-Furoic acid
98%
Synonym(s):
Furan-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H4O3
CAS Number:
Molecular Weight:
112.08
Beilstein:
110149
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
230-232 °C (lit.)
mp
128-132 °C (lit.)
SMILES string
OC(=O)c1ccco1
InChI
1S/C5H4O3/c6-5(7)4-2-1-3-8-4/h1-3H,(H,6,7)
InChI key
SMNDYUVBFMFKNZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Some of the applications of 2-furoic acid include:
- Synthesis of a single-molecule magnet, Mn11Gd2 based high-nuclearity heterometallic complex.
- Synthesis of orally active antidiabetic vanadyl complex, bis(α-furancarboxylato)oxovanadium(IV).
- As a ligand in the synthesis of luminescent 4f-3d heterometallic one-dimensional coordination polymers.
- Synthesis of biocompatible multifunctional dextran furoate nanospheres.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1C
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
282.7 °F - closed cup
Flash Point(C)
139.3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Syntheses, crystal structure and luminescence property of novel 4f-3d heterometallic one-dimensional coordination polymers.
Yin M.
Journal of Physics and Chemistry of Solids, 67(7), 1372-1378 (2006)
A new orally active antidiabetic vanadyl complex-bis (α-furancarboxylato) oxovanadium (IV).
Xie M.
Journal of Inorganic Biochemistry, 99(2), 546-551 (2005)
A bell-shaped Mn11Gd2 single-molecule magnet.
Mereacre VM.
Journal of the American Chemical Society, 129(30), 9248-9249 (2007)
Structure Design of Multifunctional Furoate and Pyroglutamate Esters of Dextran by Polymer?Analogous Reactions.
Hornig S.
Macromolecular Bioscience, 7(3), 297-306 (2007)
Jin-Xin Gao et al.
Phytopathology, 104(4), 332-339 (2013-10-19)
The maize pathotype Cochliobolus lunatus causes Curvularia leaf spot by producing a non-host-specific toxin known as methyl 5-(hydroxymethyl) furan-2-carboxylate (M5HF2C). However, related research that explores the genes that control the production of this toxin is rare. In the current work
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service