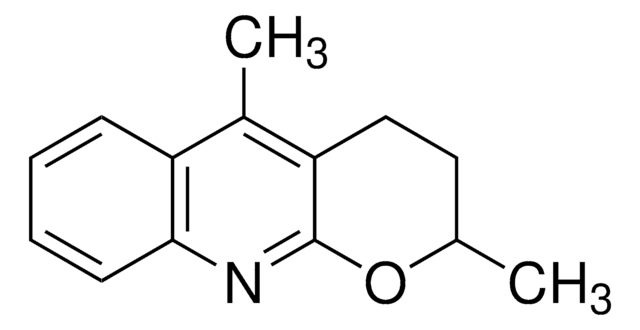
ALD00606
Wang−Yu non-directed C−H functionalization ligand
95%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H3F6NO
CAS Number:
Molecular Weight:
231.10
MDL number:
UNSPSC Code:
12352200
NACRES:
NA.22
Recommended Products
Assay
95%
form
powder or crystals
reaction suitability
reaction type: C-C Bond Formation
reagent type: catalyst
reaction type: C-H Activation
mp
145 °C
storage temp.
−20°C
Application
This 2-pyridone ligand developed in the laboratory of Jin-Quan Yu binds to palladium and accelerates non-directed C-H functionalization. Developed for C-H olefination and carboxylation with arene as the limiting reagent, the Wang-Yu ligand enables diversification of drugs, synthetic intermediates, and other bioactive small molecules.
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jin Lin et al.
Nature communications, 8, 14353-14353 (2017-02-07)
Catalytic oxidative C-H bond functionalization reactions that proceed without requiring stoichiometric amounts of external oxidants or pre-functionalized oxidizing reagents could maximize the atom- and step-economy in chemical syntheses. However, such a transformation remains elusive. Here, we report that a photo-driven
Peng Wang et al.
Nature, 551(7681), 489-493 (2017-11-24)
The directed activation of carbon-hydrogen bonds (C-H) is important in the development of synthetically useful reactions, owing to the proximity-induced reactivity and selectivity that is enabled by coordinating functional groups. Palladium-catalysed non-directed C-H activation could potentially enable further useful reactions
Direct perfluoroalkylation including trifluoromethylation of aromatics with perfluoro carboxylic acids mediated by xenon difluoride.
Tanabe Y, et al.
The Journal of Organic Chemistry, 53 (19), 4582?4585-4582?4585 (1988)
Peng Wang et al.
Angewandte Chemie (International ed. in English), 56(18), 5125-5129 (2017-04-04)
Meta-C-H functionalization of benzylamines has been developed using a PdII /transient mediator strategy. Using 2-pyridone ligands and 2-carbomethoxynorbornene (NBE-CO2 Me) as the mediator, arylation, amination, and chlorination of benzylamines are realized. This protocol features a broad substrate scope and is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)