ALD00344
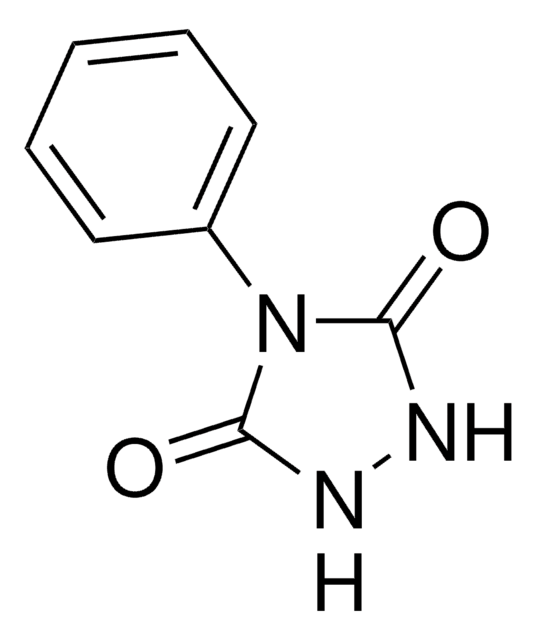
PTAD-Azide
95%
Synonym(s):
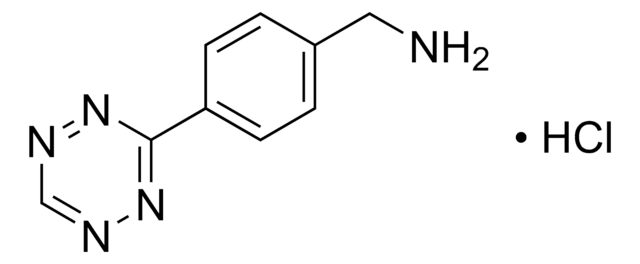
4-(4-(2-Azidoethoxy)phenyl)-1,2,4-triazolidine-3,5-dione, N3-Ph-Ur for e-Y-CLICK
About This Item
Recommended Products
Assay
95%
form
powder or crystals
reaction suitability
reagent type: cross-linking reagent
functional group
azide
storage temp.
2-8°C
SMILES string
O=C(NNC1=O)N1C2=CC=C(OCCN=[N+]=[N-])C=C2
InChI
1S/C10H10N6O3/c11-15-12-5-6-19-8-3-1-7(2-4-8)16-9(17)13-14-10(16)18/h1-4H,5-6H2,(H,13,17)(H,14,18)
InChI key
MHGMHPVYCVQIET-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Note: PTAD-Azide must be first activated by stirring in a 1:0.98 molar ratio with 1,3-dibromo-5,5-dimethylhydantoin (product # 157902). Activation is evident upon solution color change from colorless to deep red and the activated reagent should be used immediately.
General Procedure for Protein Modification with PTAD.
Part 1: PTAD activation
- Mix together 1:0.98 molar equivalents of unactivated PTAD to 1,3-dibromo-5,5-dimethylhydantoin (product # 157902) in organic solvent (preferred solvents are DMF or acetonitrile, avoid using DMSO)
- Color change is observed from colorless/pale yellow to deep red (approximately 5 min of mixing).
- After the solution turns red, store the now activated reagent on ice and use for protein modification within 30 min.
Part 2: Protein modification
- Add protein solution in mixed phosphate/Tris buffer or Tris buffer (pH should be 6 - 9) to the eppendorf tube (or other vial) containing the activated PTAD reagent prepared above and mix gently at room temperature for up to 30 min. Preferably use 10-fold molar excess of reagent relative to protein. Use protein at a minimum concentration of 1 mg/ml (higher concentrations are preferred for enhanced labeling).
- Remove excess unreacted PTAD by gel filtration.
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service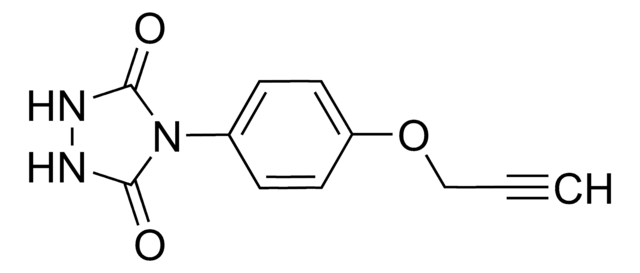




![LC-SPDP (succinimidyl 6-[3(2-pyridyldithio)propionamido]hexanoate)](/deepweb/assets/sigmaaldrich/product/structures/300/586/d95fd80c-e201-4b0b-8aee-31e109c2ff41/640/d95fd80c-e201-4b0b-8aee-31e109c2ff41.png)