752924
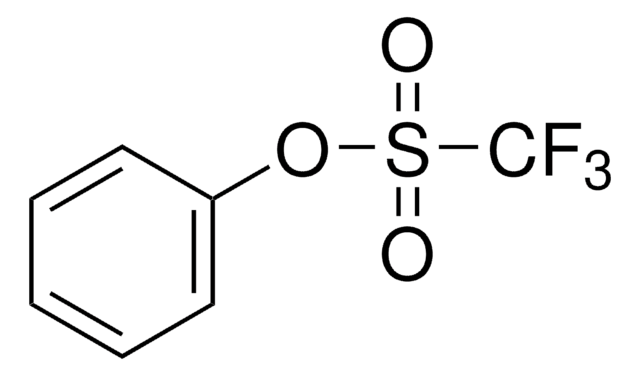
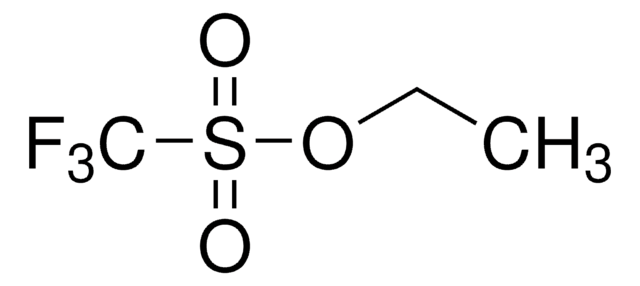
2,2,2-Trifluoroethyl trifluoromethanesulfonate
95%
Synonym(s):
Trifluoromethanesulfonic acid 2,2,2-trifluoroethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H2F6O3S
CAS Number:
Molecular Weight:
232.10
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.306
density
1.611 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
FC(F)(F)COS(=O)(=O)C(F)(F)F
InChI
1S/C3H2F6O3S/c4-2(5,6)1-12-13(10,11)3(7,8)9/h1H2
InChI key
RTMMSCJWQYWMNK-UHFFFAOYSA-N
Application
Reagent used in thenantioselective preparation of cyclic N-aryl hydroxamic acids via phase-transfer catalyzed alkylation of nitrobenzyl bromides to give nirophenylalanines
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 3 Oral - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Laura A McAllister et al.
The Journal of organic chemistry, 76(9), 3484-3497 (2011-04-02)
We describe a generalized approach to stereocontrolled synthesis of substituted cyclic hydroxamic acids (3-amino-1-hydroxy-3,4-dihydroquinolinones) by selective reduction of substituted 2-nitrophenylalanine substrates. Compounds in this series have antibacterial properties and have also recently been reported as KAT II inhibitors. The key
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)