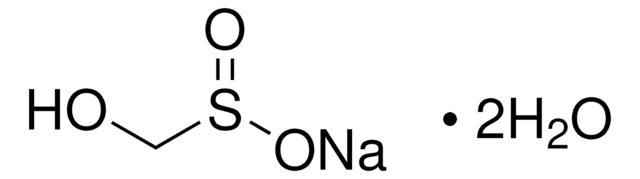71530
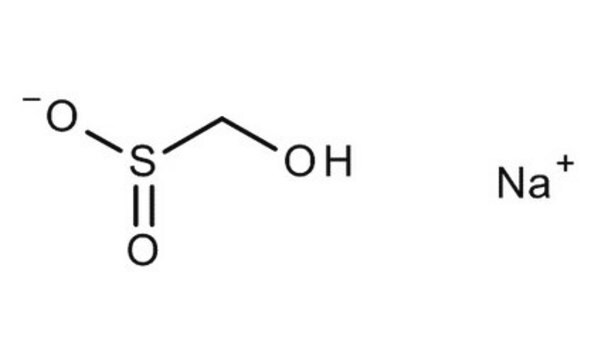
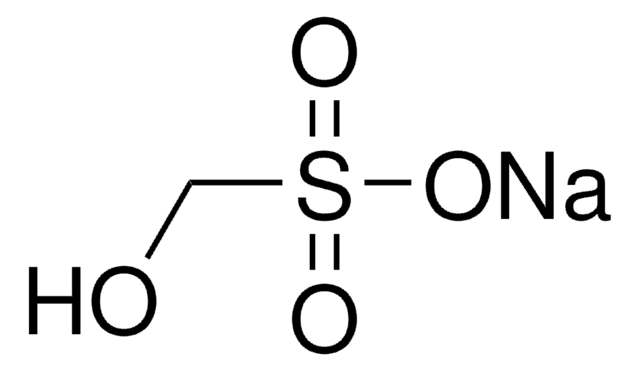
Sodium hydroxymethanesulfinate dihydrate
≥98.0% (RT)
Synonym(s):
Rongalite, Sodium formaldehydesulfoxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Quality Level
Assay
≥98.0% (RT)
form
powder
pH
9.5-10.5
solubility
H2O: 50 mg/mL, clear, colorless
functional group
sulfinic acid
SMILES string
OCS(O[Na])=O.[H]O[H].[H]O[H]
InChI
1S/CH3O3S.Na.2H2O/c2-1-5(3)4;;;/h1H2,(H,3,4);;2*1H2/q-1;+1;;
InChI key
VJWVWUHJLZEHOU-UHFFFAOYSA-N
Application
Sodium hydroxymethanesulfinate dihydrate is a versatile reagent that can be used for a wide range of organic transformations such as:
- A SO2-2anion source for the preparation of sulfones and sultines.
- Debromination of vicinal dibromoalkanes.
- Reductive dehalogenation of aldehydes and ketones.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Muta. 2 - Repr. 2
Supplementary Hazards
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rongalite: a useful green reagent in organic synthesis.
Kotha S, et al.
Chemical Reviews, 112(3), 1650-1680 (2011)
S V Makarov et al.
Archives of biochemistry and biophysics, 367(2), 289-296 (1999-07-09)
Sodium hydroxymethanesulfinate, (HOCH2SO2Na, HMS) is relatively stable in aqueous alkaline environments, but rapidly decomposes in acidic medium to give a variety of products that include sulfur dioxide. A detailed kinetic and mechanistic study of the decomposition of HMS in slightly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service