640042
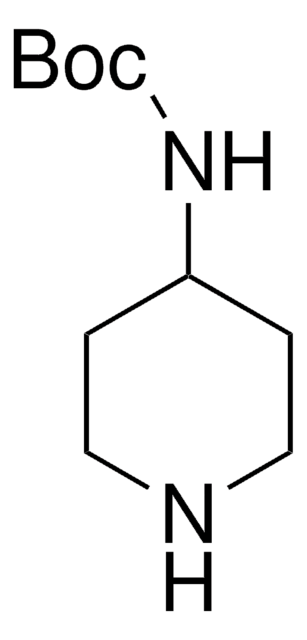
4-Amino-1-Boc-piperidine
97%
Synonym(s):
1-Boc-4-piperidinamine, tert-Butyl 4-amino-1-piperidinecarboxylate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H20N2O2
CAS Number:
Molecular Weight:
200.28
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
SMILES string
CC(C)(C)OC(=O)N1CCC(N)CC1
InChI
1S/C10H20N2O2/c1-10(2,3)14-9(13)12-6-4-8(11)5-7-12/h8H,4-7,11H2,1-3H3
InChI key
LZRDHSFPLUWYAX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Amino-1-Boc-piperidine can be used:
- as a starting material in the synthesis of N-(3-(4-hydroxyphenyl)-propenoyl)-amino acid tryptamides, which can act as silent information regulator human type 2 (SIRT2) inhibitors
- to synthesize piperidine-substituted triazine derivativesand piperidinylamino-diarylpyrimidine (pDAPY) derivatives as potent HIV-1 non-nucleoside reverse transcriptase inhibitors (HIV-1 NNRTIs)
Employed in a microwave-assisted solid-phase synthesis of N-substituted piperidines via direct annulation of primary amines with resin-bound dimesylates.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
"Synthesis and biological evaluation of piperidine-substituted triazine derivatives as HIV-1 non-nucleoside reverse transcriptase inhibitors"
Chen X, et al.
European Journal of Organic Chemistry, 51, 60-66 (2012)
"Novel piperidinylamino-diarylpyrimidine derivatives with dual structural conformations as potent HIV-1 non-nucleoside reverse transcriptase inhibitors"
Chen X, et al.
Bioorganic & Medicinal Chemistry, 23(24), 6593-6597 (2013)
"N-(3-(4-Hydroxyphenyl)-propenoyl)-amino acid tryptamides as SIRT2 inhibitors"
Kiviranta.HP, et al.
Bioorganic & Medicinal Chemistry, 17(09), 2448-2451 (2007)
Calum Macleod et al.
Journal of combinatorial chemistry, 8(1), 132-140 (2006-01-10)
The microwave-assisted solid-phase synthesis of piperazines, 3,9-diazaspiro[5.5]undecanes and 2,9-diazaspiro[5.5]undecanes is reported. The synthesis relies on the direct annulation of primary amines with resin-bound bismesylates. Critical to the success of this chemistry was the development of alpha-methyl benzyl carbamate resin linker.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service