557749
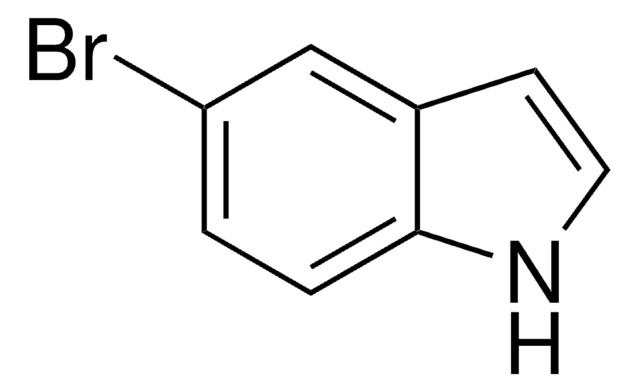
N-Boc-5-bromoindole
97%
Synonym(s):
tert-Butyl 5-bromoindole-1-carboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H14BrNO2
CAS Number:
Molecular Weight:
296.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
56-57 °C (lit.)
functional group
bromo
SMILES string
CC(C)(C)OC(=O)n1ccc2cc(Br)ccc12
InChI
1S/C13H14BrNO2/c1-13(2,3)17-12(16)15-7-6-9-8-10(14)4-5-11(9)15/h4-8H,1-3H3
InChI key
PBWDRTGTQIXVBR-UHFFFAOYSA-N
Related Categories
General description
N-Boc-5-bromoindole can undergo Sonogashira coupling reaction with N,N-diisopropylprop-2-ynylamine to afford the corresponding propargylic diisopropylamine. N-Boc-5-bromoindole is formed as an intermediate during the synthesis of 2-(5-substituted-1H-indol-3-yl)-N-hydroxyacetamide derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hiroyuki Nakamura et al.
The Journal of organic chemistry, 70(6), 2357-2360 (2005-03-12)
[reaction: see text] Propargylic diisopropylamines containing heterocycles, which were prepared readily from heterocyclic bromides and propargyldiisopropylamine by the Sonogashira coupling reaction, underwent the allene transformation reaction in the presence of Pd(2)(dba)(3).CHCl(3) catalyst (2.5 mol %) and 1,2-bis[bis(pentafluorophenyl)phosphino]ethane (10 mol %)
Sylvain Petit et al.
ChemMedChem, 4(2), 261-275 (2008-12-05)
The lead compound 5-bromoindolyl-3-acetohydroxamic acid (10) was recently identified as a potent inhibitor of bacterial peptide deformylases (PDFs). The synthesis and associated activities of new variants were investigated at position 5 to optimize the fit at the S1' subsite and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service