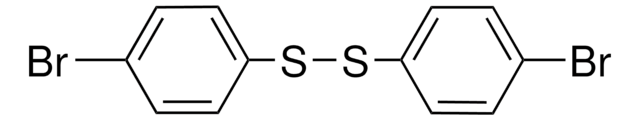557161
Bis(4-chlorophenyl) disulfide
97%
Synonym(s):
4,4′-Dichlorodiphenyl disulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[ClC6H4S]2
CAS Number:
Molecular Weight:
287.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
71-74 °C (lit.)
SMILES string
Clc1ccc(SSc2ccc(Cl)cc2)cc1
InChI
1S/C12H8Cl2S2/c13-9-1-5-11(6-2-9)15-16-12-7-3-10(14)4-8-12/h1-8H
InChI key
ZIXXRXGPBFMPFD-UHFFFAOYSA-N
General description
Bis(4-chlorophenyl) disulphide can be synthesized from 4-chlorophenylthiol via oxidation. It produces poly(p-phenylene sulfide), via thermolysis. Bis(4-chlorophenyl) disulfide can also be prepared by a microwave assisted method involving the reaction between respective elemental sulfur and 1-chloro-4-iodobenzene in the presence of CuO nanopowder (catalyst).
Application
Bis(4-chlorophenyl) disulfide may be used to synthesize non-symmetrical heterodimer 4-chlorophenyl-2′-nitrophenyl disulfide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of poly (p-phenylene sulfide) by thermolysis of bis (4-halophenyl) disulfides.
Wang ZY and Hay AS.
Macromolecules, 24(1), 333-335 (1991)
A mild and environmentally benign oxidation of thiols to disulfides.
Kirihara M, et al.
Synthesis, 2007(21), 3286-3289 (2007)
Microwave-assisted one-pot synthesis of symmetrical diselenides, ditellurides and disulfides from organoyl iodides and elemental chalcogen catalyzed by CuO nanoparticles.
Botteselle GV, et al.
J. Mol. Catal. A: Chem., 365, 186-193 (2012)
Tomislav Friščić
Chemical Society reviews, 41(9), 3493-3510 (2012-03-01)
Mechanochemical reactions effected by milling or grinding are an attractive means to conduct chemical reactions dependent on molecular recognition and to systematically explore different modes of molecular self-assembly. The natural relationship between milling mechanochemistry and supramolecular chemistry arises primarily from
Szymon Sobczak et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 24(35), 8769-8773 (2018-04-21)
This work describes, for the first time, the application of combined pressure and temperature stimuli in disulfide metathesis reactions. In the system studied, above a pressure of 0.2 GPa, equimolar amounts of symmetric disulfides bis 4-chlorophenyl disulfide [(4-ClPhS)2 ] and bis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service