523062
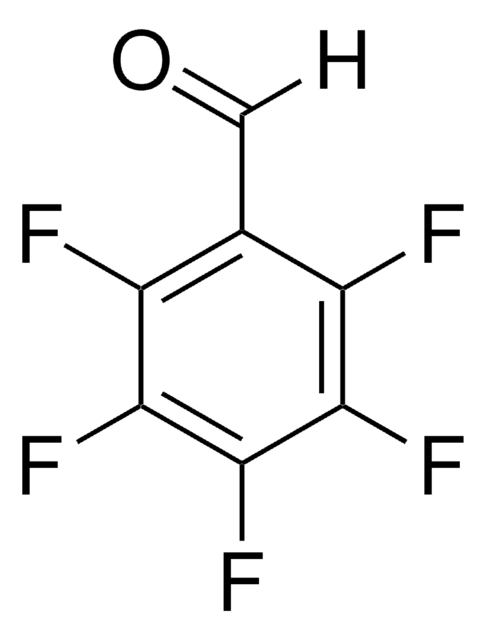
3-Vinylbenzaldehyde
97%
Synonym(s):
3-Ethenylbenzaldehyde, 3-Formylstyrene, m-Formylstyrene, m-Vinylbenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2C=CHC6H4CHO
CAS Number:
Molecular Weight:
132.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.58 (lit.)
density
1.04 g/mL at 25 °C (lit.)
functional group
aldehyde
storage temp.
2-8°C
SMILES string
[H]C(=O)c1cccc(C=C)c1
InChI
1S/C9H8O/c1-2-8-4-3-5-9(6-8)7-10/h2-7H,1H2
InChI key
CATOVPRCMWIZLR-UHFFFAOYSA-N
Related Categories
General description
3-Vinylbenzaldehyde (3-VBAL) contains a vinyl group bonded to the aromatic ring of benzaldehyde. Pulsed plasma polymerization of 3-VBAL has been described.
Application
3-Vinylbenzaldehyde may be used in the preparation of:
- 2-(3-vinylphenyl)-1,3-dioxolane
- various substituted imines
- monomer bearing a 2,4,5-triphenylimidazole moiety
- poly(3-vinylbenzaldehyde-co-dimethylacrylamide) (PVBA-co-PDMA) copolymers
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
210.0 °F - closed cup
Flash Point(C)
98.9 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A novel de-cross-linking system from cross-linked polymer to linear polymer utilizing pressure or visible light irradiation.
Iwamura T and Sakaguchi M.
Macromolecules, 41(23), 8995-8999 (2008)
Laura L Santos et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(33), 8196-8203 (2009-07-18)
Substituted imines, alpha,beta-unsaturated imines, substituted secondary amines, and beta-amino carbonyl compounds have been synthesized by means of new cascade reactions with mono- or bifunctional gold-based solid catalysts under mild reaction conditions. The related synthetic route involves the hydrogenation of a
R P Garrod et al.
Langmuir : the ACS journal of surfaces and colloids, 23(2), 689-693 (2007-01-11)
A simple two-step plasmachemical methodology is outlined for the fabrication of microcondensor surfaces. This comprises the creation of a superhydrophobic background followed by pulsed plasma deposition of a hydrophilic polymer array. Microcondensation efficiency has been explored in terms of the
Protection and polymerization of functional monomers. 15. Anionic living polymerizations of 2-(3-vinylphenyl)-1, 3-dioxolane and related monomers.
Ishizone T, et al.
Macromolecules, 24(7), 1449-1454 (1991)
New benzylidene oxazolone derived polymeric photoswitches for light-induced tunable thermoresponsive behaviors.
Balamurugan A and Lee H.
Polym. Chem., 5(22), 6426-6430 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service