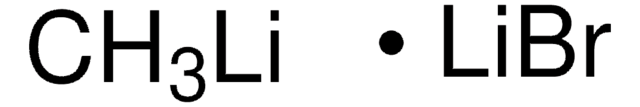514330
Methyllithium solution
3.1 M in diethoxymethane
Synonym(s):
Lithium methanide, MeLi
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
CH3Li
CAS Number:
Molecular Weight:
21.98
Beilstein:
3587162
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
concentration
3.1 M in diethoxymethane
density
0.846 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
[Li]C
InChI
1S/CH3.Li/h1H3;
InChI key
DVSDBMFJEQPWNO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Methyllithium solution ( 3.1 M in diethoxymethane) can undergo 1,2-addition to benzaldehyde catalyzed by a 2-(2-hydroxyaryl)alcohol (HAROL) ligand in the presence of titanium isopropoxide to form (S)-1-phenylethan-1-ol.
Packaging
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
Caution
Product might have solid or sediment in the bottom of the bottle.
Legal Information
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1B - Water-react 1
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flash Point(F)
5.0 °F - closed cup
Flash Point(C)
-15 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chiral 2-(2-hydroxyaryl) alcohols (HAROLs) with a 1, 4-diol scaffold as a new family of ligands and organocatalysts.
Dilek O, et al.
Tetrahedron, 74(2), 268-286 (2018)
Erik Selander et al.
Proceedings of the National Academy of Sciences of the United States of America, 112(20), 6395-6400 (2015-04-29)
Interactions among microscopic planktonic organisms underpin the functioning of open ocean ecosystems. With few exceptions, these organisms lack advanced eyes and thus rely largely on chemical sensing to perceive their surroundings. However, few of the signaling molecules involved in interactions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service