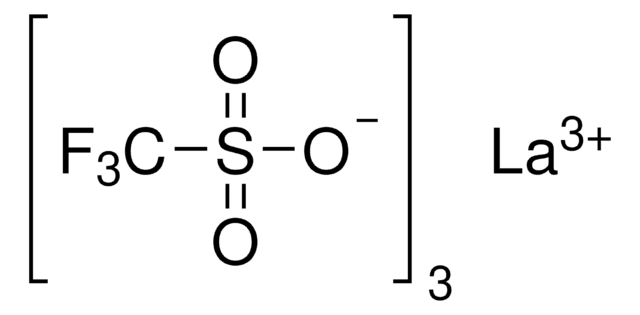
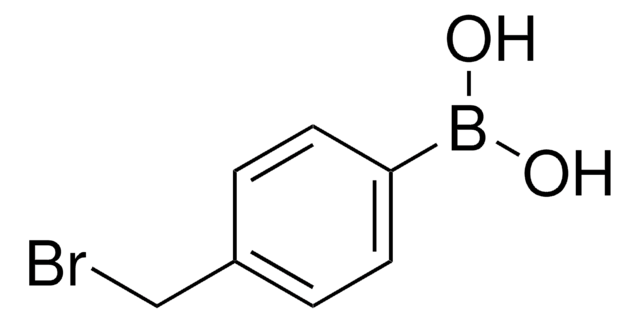
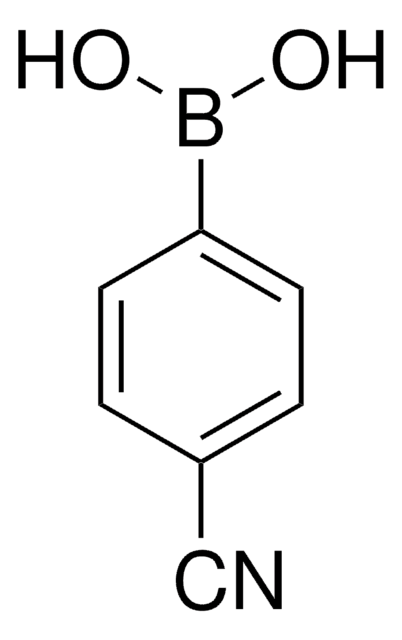
471933
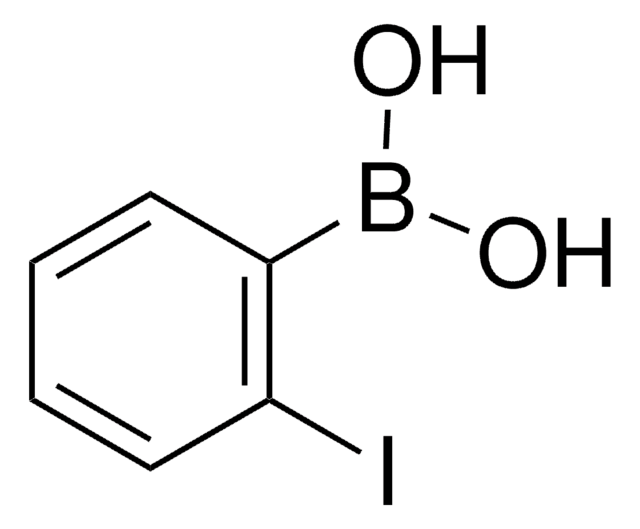
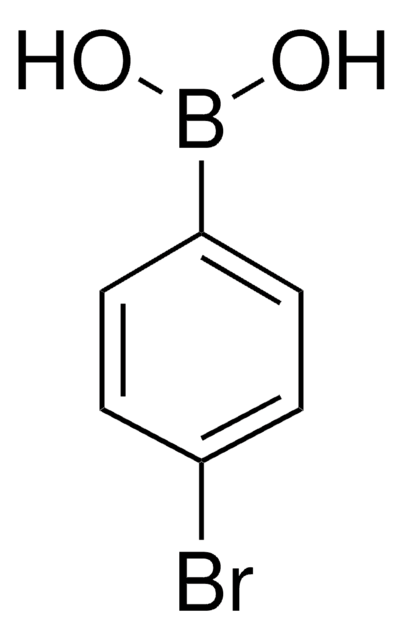
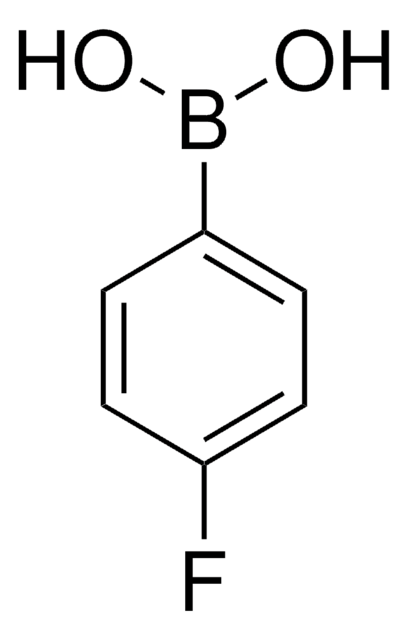
4-Iodophenylboronic acid
≥95.0%
Synonym(s):
B-(4-iodophenyl)-boronic acid, p-Iodophenylboronic acid, p-iodo-benzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
IC6H4B(OH)2
CAS Number:
Molecular Weight:
247.83
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
mp
326-330 °C (lit.)
SMILES string
OB(O)c1ccc(I)cc1
InChI
1S/C6H6BIO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
InChI key
PELJYVULHLKXFF-UHFFFAOYSA-N
Application
Reagent used for
Reagent used in Preparation of
- Copper-mediated ligandless aerobic fluoroalkylation
- Palladium-catalyzed aerobic oxidative cross-coupling reactions
- Recyclable magnetic-nanoparticle-supported palladium catalyst for the Suzuki coupling reactions
- Oxidative hydroxylation using a copper (Cu) catalyst
- Ligand-free palladium-catalyzed Suzuki-Miyaura cross-coupling
- Homocoupling using gold salts as a catalyst
- Ruthenium (Ru)-catalyzed cross-coupling
- CuI-catalyzed Suzuki coupling reactions
- Palladium-catalyzed domino Heck-Mizoroki/Suzuki-Miyaura reactions
- Manganese triacetate-mediated radical additions of arylboronic acids to alkenes
Reagent used in Preparation of
- Pleuromutilin derivatives for ribosomal binding and antibacterial activity via "Click Chemistry"
- Liquid crystalline polyacetylene derivatives
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Samir Yahiaoui et al.
The Journal of organic chemistry, 76(8), 2433-2438 (2011-03-23)
A palladium(II)-catalyzed Heck-Mizoroki/Suzuki-Miyaura domino reaction involving metal coordinating dimethylaminoethyl vinyl ethers and a number of electron-rich and electron-deficient arylboronic acids has been developed. Through variation of the temperature and the concentration of the p-benzoquinone (p-Bq) ligand/reoxidant, conditions for the robust
An efficient and recyclable magnetic-nanoparticle-supported palladium catalyst for the Suzuki coupling reactions of organoboronic acids with alkynyl bromides
X. Zhang, et al.,
Synthesis, 18, 2975-2983 (2011)
Hong Li et al.
Chemical communications (Cambridge, England), 47(27), 7880-7882 (2011-06-11)
The first ruthenium-catalyzed cross-coupling of aldehydes with arylboronic acids is reported. Various aliphatic and aromatic aldehydes are transformed to the corresponding arylketones. A total of 31 examples with moderate to excellent yields are presented, together with the results of an
Kazuhiko Tsukagoshi et al.
Journal of chromatography. A, 1123(1), 106-112 (2006-05-24)
Molecular recognition of mono- and disaccharides was performed making use of the interaction between their diol groups and p-iodophenylboronic acid in capillary electrophoresis (CE) with a chemiluminescence (CL) detection system. p-Iodophenylboronic acid acted as an enhancer for luminol-horseradish peroxidase-hydrogen peroxide
Disubstituted Liquid Crystalline Polyacetylene Derivatives That Exhibit Linearly Polarized Blue and Green Emissions
San Jose, B. A.; et al.
Macromolecules, 44, 6288-6302 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service