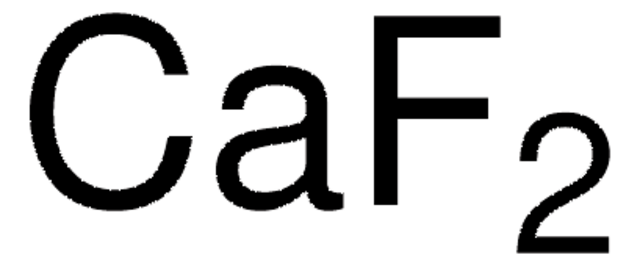449717
Calcium fluoride
anhydrous, powder, 99.99% trace metals basis
Synonym(s):
Fluorite, Fluorspar
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
CaF2
CAS Number:
Molecular Weight:
78.07
EC Number:
MDL number:
UNSPSC Code:
12352302
eCl@ss:
38050110
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
grade
anhydrous
Quality Level
Assay
99.99% trace metals basis
form
powder
impurities
≤150.0 ppm Trace Metal Analysis
bp
2500 °C (lit.)
density
3.18 g/mL at 25 °C (lit.)
SMILES string
F[Ca]F
InChI
1S/Ca.2FH/h;2*1H/q+2;;/p-2
InChI key
WUKWITHWXAAZEY-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
- Calcium fluoride based multifunctional nanoparticles for multimodal imaging - This study explores the integration of ions in the calcium fluoride lattice for enhanced multimodal imaging applications (M Straßer et al., 2017).
- Preparation and characterization of calcium fluoride nano particles for dental applications - Discusses the preparation of nano-sized calcium fluoride for use in dental composites, aiming to improve dental fillings (MS Al-Ajely et al., 2018).
- Optimization of Calcium Fluoride Crystallization Process for Treatment of High-Concentration Fluoride-Containing Semiconductor Industry Wastewater - Examines the optimization of calcium fluoride crystallization for treating industrial wastewater (A Sinharoy et al., 2024).
- Synergetic strengthening mechanism of ultrasound combined with calcium fluoride towards vanadium extraction from low-grade vanadium-bearing shale - Investigates the effects of combining ultrasound and calcium fluoride on the extraction of vanadium from shale (B Chen et al., 2021).
- Sulfate Doping Promotes Agglomeration of Calcium Fluoride Crystals - Studies the influence of sulfate doping on the agglomeration of calcium fluoride crystals, important for industrial applications (T Wang et al., 2024).
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bioceram. Proc. Int. Symp. Ceram. Med., 7, 279-279 (1994)
Australian Journal of Chemistry, 49, 897-897 (1996)
Jason S Lupoi et al.
Biotechnology for biofuels, 8, 98-98 (2015-07-23)
Slow-degrading, fossil fuel-derived plastics can have deleterious effects on the environment, especially marine ecosystems. The production of bio-based, biodegradable plastics from or in plants can assist in supplanting those manufactured using fossil fuels. Polyhydroxybutyrate (PHB) is one such biodegradable polyester
J M ten Cate
European journal of oral sciences, 105(5 Pt 2), 461-465 (1997-12-12)
Low concentrations of fluoride have a beneficial effect on enamel and dentin de- and remineralization. After fluoride treatments, such as topical applications, rinses or dentifrices, salivary fluoride concentrations decrease exponentially in a biphasic manner to very low concentrations within a
G Rølla
Acta odontologica Scandinavica, 46(6), 341-345 (1988-12-01)
The literature concerning the formation and stability of CaF2 in the oral environment is reviewed. In early work the CaF2 formed during topical application with fluoride was assumed to be beneficial. It was suggested that it could protect the enamel
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service